Diabetes is a worldwide health problem, and its prevalence is expected to rise in the coming years, not only in adults, but also in youth. The first stage is characterized by insulin resistance accompanied by a compensatory increase in insulin secretion; this stage can last several years, and it is called prediabetes. Patients with both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) have insulin resistance, but the site of their predominant insulin resistance differs.1,2 Prediabetes is defined by the American Diabetes Association (ADA) as a fasting glucose concentration of 100–124 mg/dl, or a HbA1c value of between 5.7% and 6.4%, or a two-hour post-glucose tolerance concentration of 140–199 mg/dl. Therefore, prediabetes includes subjects with elevated fasting plasma glucose concentrations—IFG, and normal response to a glucose load, normal glucose tolerance, subjects with abnormal postprandial glucose excursion—IGT, but normal fasting glucose concentration, or a combination of the aforementioned.3 Those with IFG have predominantly hepatic insulin resistance, whereas those with IGT have predominantly muscle insulin resistance. The increased prevalence of obesity and diabetes mellitus dictates increased awareness and early identification of diabetes.
In Saudi Arabia, the prevalence of prediabetes was equal to 5.4%, based on oral glucose tolerance test (OGTT), and 21.9% based on HbA1c.4 According to epidemiological data from the UK, the prevalence rate of prediabetes in the general population increased from 11.6% to 35.3% from 2003 to 2011.5 An increase in prediabetes prevalence has also been observed in China, where prediabetes has risen to 15.5% in the general population.6 In a national cohort study of a representative sample of adolescents, it was estimated that the national population-based prevalence rates of IFG, IGT, and prediabetes among US adolescents aged 12–19 years were 13.1%, 3.4%, and 16.1%, respectively.7 In a recent report from the US, the prevalence of prediabetes increased from 9% to 23%.8 Worldwide, epidemiological trends demonstrate increases in the prevalence of prediabetes in the general population; adolescents seem to follow these trends, irrespectively of race. One of the most important and difficult stages in life is the transition from childhood to adulthood. This stage in life is called adolescence. It is characterized by various physical, emotional, social, and sexual developments. It is well established that the first two kinds of changes are due to developmental hormonal changes occurring during adolescence. These hormonal changes have been shown to be responsible for various effects on glucose metabolism.9,10
One of the most important and difficult stages in life is the transition from childhood to adulthood. This stage in life is called adolescence. It is characterized by various physical, emotional, social, and sexual developments. It is well established that the first two kinds of changes are due to developmental hormonal changes occurring during adolescence. These hormonal changes have been shown to be responsible for various effects on glucose metabolism.9,10
Because of a disturbing increase in obesity in adolescence over the past two decades, prediabetes and metabolic syndrome in adolescents have become major public health problems.11 However, screening and prevention of prediabetes in adolescence is often overlooked, leading to an increased rate of young adults with type 2 diabetes mellitus and metabolic syndrome. Much debate has taken place concerning the cut-off values of the revised impaired fasting glucose concentration levels.12 There is still no clear consensus on the definition of prediabetes, or metabolic syndrome in adolescents. We use the criteria of prediabetes in adults. However, there are only a few observational studies about prediabetes in adolescence.
In this review, we intend to unravel the associations between the endocrine physiology of adolescence and prediabetes, summarizing all the findings, and aspiring to contribute to the awareness of prediabetes during adolescence. We performed systematic research using PubMed, EBSCO, Google Scholar databases and ResearchGate site, in relation to adolescence and prediabetes.
Glucose and insulin metabolism in adolescence
Adolescence is characterized by the domination of luteinizing hormone (LH) concentrations over those of the follicle-stimulating hormone (FSH) under the stimulating effect of the hypothalamic gonadotropin-releasing hormone (GnRH). These changes lead to the secretion of sex steroids, namely estradiol in females and testosterone in males; development of secondary sexual characteristics; growth acceleration; lean and fat mass accumulation; and observed growth spurt. During this phase, a well-described physiological decrease in insulin sensitivity occurs, which resolves after completion of puberty, possibly independently of changes in body mass index (BMI).
Onset of adolescence, Tanner staging, and glucose metabolism
GnRH resides under the control of kisspeptin, its permissive neurokinin B, and its opposing dynorphin signals.13 GnRH does not seem to be implicated in glucose homeostasis derangement. On the contrary, the hypothalamic system of kisspeptins is implicated in the secretion of insulin, as they are highly abundant in the pancreas.14 Moreover, kisspeptins seem to have a protective role in gestational diabetes onset.15 On the other hand, it has been shown that ablation of dynorphin causes increase of fatty acid oxidation in male mice, reduction in adiposity, and increased weight loss during fasting, probably due to decreases in intestinal nutrient absorption.16 There has been no study performed about neurokinin B in relation with insulin resistance, or glucose intolerance, and dynorphin does not seem to essentially affect glucose homeostasis.17 Dynorphin seems to be implicated in the pathogenesis of obesity,18 and thus it affects only indirectly the pathogenesis of prediabetes in susceptible subjects.
To our knowledge, no study exists that examines the role of sex steroids in association with prediabetes in adolescents. Only one study19 examined LH in relation with glucose metabolism impairment in postmenopausal women who had received no hormone replacement therapy. In this study, it was found that the decreased FSH concentrations were positively associated with the presence of prediabetes and diabetes. This finding was attributed to the concurrent positive association of FSH concentrations, and inflammatory markers, such as C-reactive protein, tumor necrosis factor-alpha (TNF-α) and interleukin 1 beta (IL-1β), caused by the elevated concentrations of estradiol and the decreased concentrations of sex hormone binding globulin (SHBG).
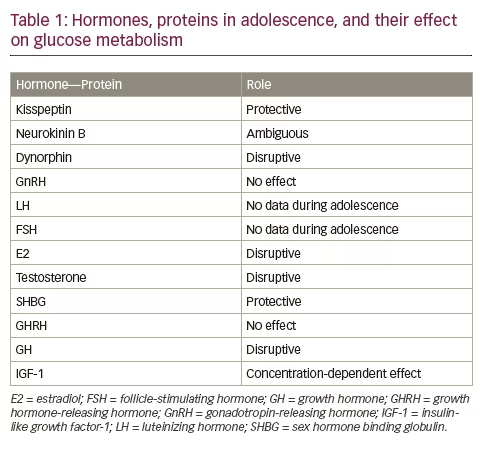
Testosterone clearly contributes in the development of insulin resistance,20 if not per se, then via indirect mechanisms.21
Pubertal stages, and homeostasis of glucose and insulin Insulin sensitivity and glucose metabolism differs in the various stages of puberty. Moran et al. showed decreased insulin sensitivity in puberty, reaching a nadir at mid-puberty, and improving in the late pubertal stage, indicating the transient nature of these changes, which were more prominent in females than in males.22,23 Only three cohort studies have assessed insulin sensitivity by individual Tanner stage, which demonstrated conflicting results. Goran et al. showed a decrease in insulin sensitivity and an increase in acute insulin response, through the pubertal stages.24 Ball et al. reported a decrement in insulin sensitivity, at onset and subsequent recovery at the end of puberty. Decreased acute insulin response, increased fat mass and insulin secretion, linearly across Tanner stages, have been shown in both Caucasian and African American participants, and increasing fat mass was also associated with increasing insulin secretion.25 In contrast, Hoffman et al. demonstrated greater insulin sensitivity in early pubertal boys than in girls, which was primarily attributed to the effects of body fat mass differences and/or peripheral growth hormone (GH) action. Insulin sensitivity in their sample was determined primarily by body mass and/or peripheral GH action.26
Growth spurt and homeostasis of glucose and insulin
Growth spurt in adolescence is the rapid and intense increase in the rate of growth in height and weight that occurs during the adolescent stage of the human life cycle. Adolescence has profound effects on the physiology of the GH/insulin-like growth factor-1 (IGF-1) axis. GH secretion increases with the onset of adolescence. The increase in GH secretion is the result of increased GH pulse amplitude rather than frequency, and is an estrogendependent effect. Parallel to the increase in GH secretion, circulating IGF-1, and IGF binding protein-3 (IGFBP-3) also increase. The increase in IGF-1 correlates with phases of adolescence, as well as sex steroid levels. Circulating immunoreactive growth hormone-releasing hormone (GHRH) also increases, while circulating GH-binding protein levels are not affected by sexual maturation.27
GH, via the action of IGFs, stimulates lipolysis, increased amino-acid transport into tissues, and increased protein and glucose synthesis in the liver. It is secreted in pulses, mainly at night, with peaks during the short intervals of the day, but it remains low during the day. During adolescence, increased pulse amplitude is observed. In excess, GH is diabetogenic, causing insulin resistance. The latter is due to increased endogenous glucose production, and decreased glucose disposal in muscle. These effects appear to be largely secondary to GH-induced lipolysis, and subsequent glucose-fatty acid substrate competition. Accumulation of lipid metabolites initiates a cascade, which inhibits phosphoinositide 3 (PI 3) -kinase activity and translocation of the glucose transporter type 4 (GLUT-4) glucose transporter to the cell surface.28
On the other hand, normal IGF-1 concentrations seem to protect from the diabetogenic effects of GH,29 while low and high-normal IGF-1 concentrations are both associated with insulin resistance.30 Insulin and IGF-1 are capable of binding to the insulin receptor and the IGF-1 receptor, but with low affinity. The insulin sensitizing effect of IGF-1 appears to be mediated mainly by hybrid receptors that are present in large amounts in skeletal muscle. IGF-1 plays an important role in carbohydrate metabolism, maintaining a balance between the actions of insulin and GH. IGF-1, also, enhances glucose uptake into peripheral tissues, such as skeletal muscles.31
The findings of Moran et al. showed that the association of IGF-1 with fasting insulin differs between the sexes, and appears to be related to adiposity in girls. The relationship between fasting insulin and IGF-1 concentrations was significant in lean girls, but its significance decreased with increasing percentage of body fat. Fasting insulin levels had risen in response to increasing body fat until a maximal influence of fasting insulin on IGF-1 was exceeded, and a plateau effect reached, thus giving the appearance of a lessening effect with increasing fatness. In boys, however, there was no significant relationship between IGF-1 and fasting insulin and no effect of percentage body fat on that interaction.32 The aforementioned data are summarized in Table 1.
Obesity in adolescence, and glucose metabolism First, it is important to acknowledge that weight gain during puberty is normal; puberty in girls is accompanied by increase in BMI, and subcutaneous adiposity. Thus, the early onset of adolescence from any cause can produce increases in parameters frequently used to define obesity, including age-normalized BMI. Earlier thelarche was positively associated with elevated age-normalized BMIs at age three years and with increased velocity of BMI change from the age of three years to the first grade of school.33
The same trend has been observed in males. The onset and progression of adolescence seem to correlate significantly with weight and BMI. Moreover, in overweight males, pubertal development begins and proceeds to the late stage earlier in comparison with normal-weight children.34 Also of note, in a study by Paltoglou et al.35 obese, male adolescents demonstrated lower baseline concentrations of GH and increased concentrations of prooxidation markers, than those of their lean counterparts.
Thus, obesity is associated with sexual maturation in both boys and girls, but the timing and duration differs from that of normal-weight children. Girls enter adolescence earlier, and mature sexually faster than boys, who enter the adolescent stage earlier, but the duration of sexual maturation is longer.36 Positive family history of type 2 diabetes and obesity are the most important risk factors for the onset of prediabetes.37 One study found that obese adolescents, who demonstrate abnormal fasting glucose, glucose intolerance, or both, are more likely to have impaired insulin secretion, rather than reduced insulin sensitivity. Given the impairment in insulin secretion, they are at high risk for progression to type 2 diabetes.38
Obesity in adolescence has been associated with increased risk for coronary heart disease in adulthood. Moreover, Bacha et al.39 demonstrated that, irrespective of glycemic status, adiposity was the major determinant of coronary arterial calcifications; hyperglycemia, age, and race for carotid intima-media thickness; leptin and insulin sensitivity for arterial stiffness. Individuals with IGT, IFG, or both, are at high risk, not only of developing diabetes mellitus, but also of experiencing an adverse cardiovascular event later in life.40
A recent addition to the complications of obesity is non-alcoholic fatty liver disease (NAFLD).41 Insulin resistance is clearly important, acting to increase free fatty acid (FFA) influx into the liver, driving hepatic triglyceride production. The accumulation of hepatic triglycerides leads to hepatic insulin resistance by interfering with tyrosine phosphorylation of insulin receptor substrates 1 and 2.42 Several studies have established that arterial stiffness related to the presence of NAFLD is grounded on the presence of an adverse metabolic profile in adolescents.43 Last, a study by Sert et al. demonstrated that obese adolescents with NAFLD have increased left ventricular mass, carotid intima media thickness, and low insulin sensitivity.44
Current guidelines from the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) Hepatology Committee suggest that NAFLD should be suspected in all overweight or obese children, and adolescents older than three years with increased waist circumference, especially if there is a positive NAFLD family history. NAFLD presents typically in children aged 10 years and older. The first step in diagnosis should be abdominal ultrasound and liver function tests, followed by exclusion of other liver diseases. Overweight, or obese children and adolescents with normal ultra-sonographic imaging and normal liver function tests should still be monitored due to the poor sensitivity of these tests at a single assessment.45 These findings add to the accumulative data about the increased health risk for obese adolescents with prediabetes.
Behavioral and psychosocial aspects of prediabetes in adolescents
Lifestyle behaviors have a profound impact on the onset of prediabetes. Decreased dietary intake of fiber, lack of exercise, sedentary lifestyle, sleep deprivation, increased screen time, and high levels of stress contribute to disruption of glucose homeostasis, regardless of obesity levels. But the aforementioned lifestyle parameters eventually lead to obesity.46
Improper dietary intake
There is an indisputable association between unhealthy diet behaviors, such as increased junk food consumption, sweetened beverages, reduced consumption of fiber, and disrupted food daily timeline.47 The ADA recommends dietary strategies that reduce calories, and intake of dietary fat, and increase the intake of dietary fiber and foods containing whole grains, while limiting the intake of sugar-sweetened beverages.48 Many studies have pointed out the importance of dietary fibers of in daily meals.49 Several studies have also pointed out unhealthy dietary habits, observed in adolescence. In many studies, associations have been found between dietary habits and socio-economic status. Higher socio-economic status is associated with lower energy intake from snack episodes, breakfast skipping, and energy density of foods and these associations have been proven to be unchanged over time.50
Lack of exercise, increased screen time, and sedentary lifestyle Shifts from traditional to modern lifestyles over the past century, characterized by a decrease in daily physical activity, have been accompanied by a rise in the prevalence of obesity and prediabetes. Results from physical activity interventions for the prevention of type 2 diabetes provide perhaps the most direct evidence of this association, demonstrating that increased physical activity significantly reduces the risk of glucose regulation disruption, or delays the onset of type 2 diabetes, even among individuals with impaired glucose tolerance, compared to control conditions.51 Numerous studies have demonstrated the lack of exercise due to increased screen time in adolescents.52
Increased stress levels It is established knowledge that increased stress levels lead to disruptive health behaviors.53,54 During adolescence, there are various social stressors, originating either from the individual’s family, about overachievement, or from their peers, about social acceptance.55 A developmental procedure called pruning also takes place during adolescence. Adolescence is a period of susceptibility, on which stress might have adverse effects in adulthood. When adolescent brains are exposed to prolonged, or high stress levels, this may result in hyperactivity of the stress system of the brain. Hyperactivity of the brain stress system includes amygdala hyperfunction, causing excessive fear reaction; decreased activity of the hippocampus, causing defective cognition; and the meso-corticolimbic dopaminergic system, resulting in dysthymia, novelty-seeking, and addictive behaviors. On the other hand, hypothalamic–pituitary–adrenal (HPA) axis hyperactivation and subsequent hypercortisolism are accompanied by abnormal childhood, adolescent and adult behaviors, including excessive fear, and addictive behaviors, dysthymia and depression, and gradual development of components of metabolic syndrome X, including visceral obesity, essential hypertension, and ultimately intermediate hyperglycemia, or diabetes.56
Screening for prediabetes According to the ADA Statement of Standard Care, screening for diabetes, or prediabetes in children and adolescents below the age of 18 years of age should be started in overweight subjects (BMI >85th percentile for age and sex, weight for height >85th percentile, or weight >120% of ideal for height) plus any two of the following risk factors:
• Family history of type 2 diabetes in a first- or second-degree relative.
• Race/ethnicity, such as Native American, African American, Latino, Asian American, Pacific Islander.
• Signs of insulin resistance, or conditions associated with insulin resistance (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome, or small-for-gestational-age birth weight)
• Maternal history of diabetes, or gestational diabetes during the child’s gestation.
Screening for prediabetes should be started at 10 years of age, or at onset of adolescence, if adolescence occurs at a younger age. In case of negative results, the screening should be repeated every three years.57 BMI is widely used as a surrogate measure of obesity, but it underestimates the prevalence of obesity, defined as an excess of body fat. A recent study found that assessing the percentage of body fat may help to diagnose disturbed glucose tolerance beyond information provided by BMI.58 The results of another study showed that the inclusion of body composition measurements together with morbidity evaluation in the routine medical practice both for the diagnosis and the decision-making for instauration of the most appropriate treatment of obesity is desirable.59
Given that the progression of the glucose homeostasis alterations in adolescents are usually rapid and insidious,60 BMI seems to be inadequate in the assessment for prediabetes screening in adolescence. Also, in overweight and obese subjects, measuring only fasting plasma glucose concentrations and HbA1c ratio are inadequate means for prediabetes screening.61 OGTT remains the gold standard for the direct evaluation of insulin secretion, and the indirect evaluation of the degree of insulin resistance.62
Conclusions
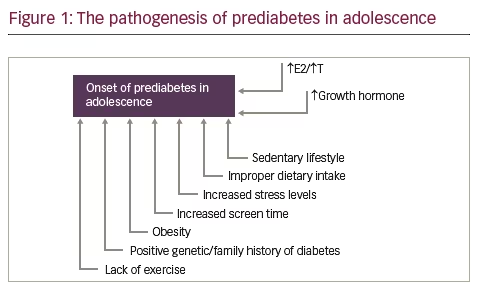
Adolescence is a period of increment in lean, fat, and bone mass, along with the advancement in sexual maturation. The physiological phenomenon of insulin resistance seems to play a pivotal role in the physical changes in body composition during adolescence. The hormonal fluctuations of puberty render adolescents prone to impaired glucose metabolism, as depicted in Figure 1. Prediabetes, either in the form of insulin resistance, or abnormal glucose tolerance remains a risk factor for cardiovascular complications, and type 2 diabetes in adulthood, especially when it is combined with additional risk factors, such as obesity, or a positive family history of diabetes. Obesity is strongly associated with prediabetes, but also visceral and increased total subcutaneous fat, even when BMI is normal for age.58 BMI does not seem to be adequate in assessment for prediabetes screening either in adolescents, or in adults.
A contemporary, sedentary lifestyle, subsequent lack of exercise, increased sweetened foods, and decreased dietary fibers have all contributed to the disturbing increase in the obesity epidemic, that has been observed for the last 30 years. New, efficient strategies must be employed to modify all parameters, that provoke impairment of glucose metabolism in adolescence. Proper screening—per ADA Standards of Care for Diabetes— including OGTT, fasting plasma glucose concentrations, and HbA1c measurement, together in association with the assessment of diabetes risk factors provide the best chance of diagnosing and treating prediabetes, and thus preventing type 2 diabetes onset, its complications, and the economic burden on healthcare systems.







