2016 has been a landmark year for diabetology. While the last decade has seen various pharmacological advances, 2016 stands out for consolidation of existing drugs. Not one, but two, glucose-lowering molecules demonstrated beneficial effects on cardiovascular health this year. These drugs have also been able to show renal safety and benefit in diabetes.
A tale of two trials
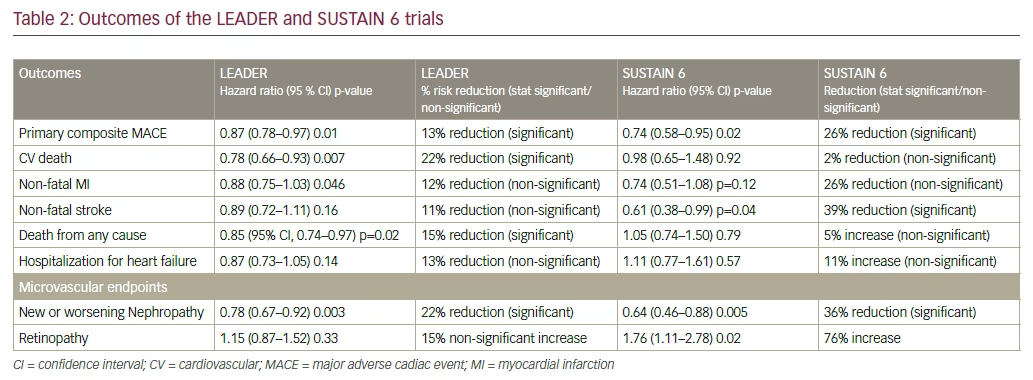
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results—A Long Term Evaluation (LEADER) trial proved the cardiovascular benefit of liraglutide when used in participants with diabetes at high risk of, or established, cardiovascular disease (CVD).1 Liraglutide use was associated with a lower risk of cardiovascular mortality and lower all-cause mortality (see Tables 1 and 2). A 13% reduction of the primary endpoint (hazard ratio [HR] 0.87; 95% confidence interval [CI], 0.78–0.97; p<0.001 for non-inferiority; p=0.01 for superiority) for liraglutide-treated compared to placebo-treated subjects. For the individual components of the primary endpoint, 22% reduction of cardiovascular death (HR 0.78; 95% CI, 0.66–0.93; p=0.007) was noted. Reduction in nonfatal stroke and non-fatal myocardial infarction (MI) was noted. Non-significant reductions of nonfatal myocardial infarction (HR 0.88; 95% CI, 0.75–1.03; p=0.11) and non-fatal stroke (HR 0.89; 95% CI, 0.72–1.11; p=0.30) were found. For the expanded cardiovascular (CV) endpoint, there was a 12% reduced risk (HR 0.88; 95% CI, 0.81–0.96; p=0.005). There was a non-significantly reduced risk of hospitalization for heart failure (HR 0.87; 95% CI, 0.73–1.05; p=0.14).
There was also a lower risk of renal events. A 16% reduction in the composite microvascular endpoint (HR 0.84; 95% CI, 0.67–0.92; p=0.02) for liraglutide-treated versus placebo-treated subjects in which 22% reduction in the risk of the composite nephropathy outcome (HR 0.78; 95% CI, 0.73–0.97; p=0.003) was demonstrated.
Liraglutide was able to reduce insulin requirement, reduce body weight, and reduce hypoglycemic episodes, while achieving lower glycated haemoglobin (HbA1c). An estimated treatment difference (ETD) in HbA1c between liraglutide and placebo of -0.40 % (95% CI -0.45 to -0.34) was observed at 36 months. Severe hypoglycemia occurred in 114 liraglutide- and 153 placebo-treated subjects, yielding a rate ratio of 0.68 (95% CI, 0.51–0.91). Similarly, the rate ratio for confirmed hypoglycemia (<56 mg/dl) was 0.80 (95% CI, 0.74–0.88), which corresponds to a 32% lower risk for severe hypoglycemia and 20% lower risk for confirmed hypoglycemia (<56 mg/dl) for liraglutide-treated subjects compared to placebo-treated subjects. An ETD in body weight between the liraglutide and placebo arm of -2.3 kg (95% CI, -2.5–-2.0) was observed at 36 months. The major part of the weight loss was achieved within the first six months of the trial, and remained stable during the rest of the trial.
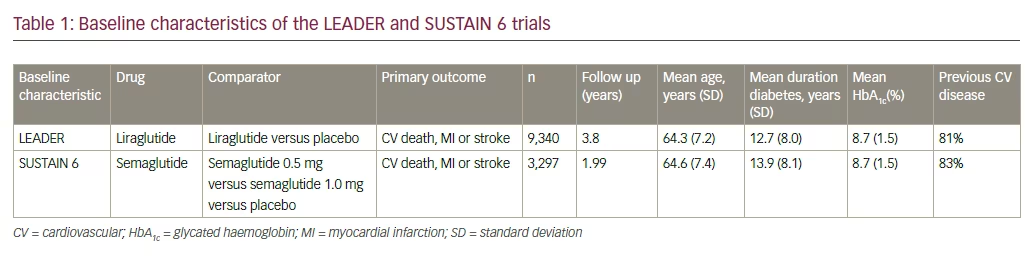
Thus, these results support the use of liraglutide as a preferred second-line drug in diabetes care, provided it is not contraindicated and is well tolerated.
The positive results seen in LEADER were repeated in the Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6), which assessed the cardiovascular outcomes of semaglutide, a once-weekly injectable glucagon-like peptide-1 receptor agonists (GLP1RA). Semaglutide use led to a statistically significant reduction in stroke, and a numerically significant fall in non-fatal MI.2 Semaglutide demonstrated 26% reduction in composite CV outcome compared with placebo. The long-acting analogue reduced the risk of non-fatal stroke by 39%, (HR = 0.61, 95% CI [0.38; 0.99] p=0.044) and non-fatal MI by 26% (HR = 0.74 95% CI [0.51; 1.08] p=0.12). Though overall mortality did not fall, reduction in HbA1c, body weight, insulin requirement and hypoglycemia were noted.
The SUSTAIN 6 results also support early use of GLP1RA in the diabetes therapeutic landscape.
Shades of gray
While both the LEADER and SUSTAIN 6 results demonstrate CV benefit, there are subtle differences in their vascular effects. Semaglutide has shown significant reduction in the risk of non-fatal MI, thus acting as a primary preventive strategy against CV events. On the other hand, liraglutide reduces the risk of all-cause mortality and CV mortality, without significantly reducing the chances of having a non-fatal MI or stroke. This implies that liraglutide works better in secondary prevention, that is, prevention of complications after an acute cardiovascular event has occurred. Semaglutide use was associated with an increase in retinal events, while there was no change noted with liraglutide. Renal events were markedly reduced with both drugs, however. Semaglutide’s benefits were visible within four to six months of the trial, while liraglutide took 12 to 18 months to demonstrate its advantage.
The differences noted between liraglutide and semaglutide may be associated with their duration of action, or with their capacity to cross the blood brain barrier and influence cerebral blood flow, either directly, or through an effect on neuronal cells and astrocytes.
Great expectations
More important, however, is the impact the trials reported in 2016 will have on future diabetes care. It is less than 20 years since the UK Prospective Diabetes Study (UKPDS) provided proof of reduction of chronic vascular outcomes with glucose-lowering therapy.3 It took another decade for regulatory authorities to ask for evidence of cardiovascular safety of anti-diabetic drugs, making cardiovascular outcome trials mandatory.4 Now LEADER and SUSTAIN 6 (along with the Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial [EMPA REG])5 have raised the bar by offering CV benefit, over and above safety, with a drug primarily meant to reduce glycemia. This sets the stage for great expectations and, hopefully, better delivery of comprehensive care in diabetes.
Sense and sensibility
The data released in 2016 will stimulate and support calls for comprehensive vasculo-metabolic care using a minimal number of drugs (therapeutic parsimony).6 Paradoxically, at the same time, the unique vascular bed-specific benefits of various antidiabetic drugs suggest that attention will focus on rational combinations of drugs to achieve panvascular safety and benefit. For example, a combination of empagliflozin and semaglutide/liraglutide may offer maximal benefit in postponing death and reducing the risk of cardiovascular events. Similarly, a combination of fenofibrate and semaglutide may provide optimal coverage against both retinopathy7 and stroke.
Treasure island
Exaptation of glucose-lowering drugs for non-glycemic purposes8 may be another spin-off from developments reported this year. Liraglutide is approved for use in obesity (in a 3.0 mg dose),9 and other uses are also being explored. Semaglutide, too, has the potential to be of utility in nondiabetic indications. The next decade may see these molecules being used not only for their glucose-lowering efficacy, but also for their vasculoprotective and metabolic modulatory effects. These uses may extend from neurological conditions such as Alzheimer’s disease to metabolic conditions like polycystic ovary syndrome.10
Summary
The year 2016 has been a landmark year in diabetology. Developments reported this year should help change the way in which we approach, and manage, not only diabetes, but all cardiovascular and metabolic disease.







