The total number of people with diabetes worldwide is projected to rise from 366 million in 2012 to 552 million in 2030.1 Optimal glycaemic control is essential to managing risks in patients with type 2 diabetes as lower glycaemic exposure is associated with a 25 % reduction in the risk of microvascular complications.2 However, despite improved therapies and medical care, glycaemic control remains poor among a large proportion of type 2 diabetes patients.3
The total number of people with diabetes worldwide is projected to rise from 366 million in 2012 to 552 million in 2030.1 Optimal glycaemic control is essential to managing risks in patients with type 2 diabetes as lower glycaemic exposure is associated with a 25 % reduction in the risk of microvascular complications.2 However, despite improved therapies and medical care, glycaemic control remains poor among a large proportion of type 2 diabetes patients.3
Historically, glycaemic control targets have emphasised fasting plasma glucose (FPG) targets, but post-prandial increases in blood glucose contribute significantly to overall glycaemic control in type 2 diabetes. Recent findings suggest that 40–50 % of patients treated with basal insulin and oral diabetic drugs do not achieve their glycated haemoglobin (HbA1c) goals, despite achieving FPG control. 4 Importantly, the post-prandial state is the norm for most patients; the true fasting state typically exists only in the 2 hours before breakfast for those who consume three meals a day at relatively regular intervals. Thus, post-prandial glucose (PPG) is increasingly becoming recognised as a therapeutic target for optimising glycaemic control in type 2 diabetes.
In recent years, recommendations for type 2 diabetes have reflected increased awareness of the importance of PPG control: the International Diabetes Federation in Europe recommends a PPG target of ≤7.5 mmol/l.5 Admittedly, debate persists over the relative importance of FPG and PPG.6 It has been suggested that setting targets for PPG is unrealistic and even unsafe because they may increase the risk of hypoglycaemia.7 However, recommendations on PPG control are supported by a considerable body of scientific data. The majority of patients with type 2 diabetes have elevated PPG, even when HbA1c is satisfactory (<7 %).8 Another study found elevated post-challenge plasma glucose (a surrogate of PPG) in 74 % of individuals with type 2 diabetes.9 Finally, PPG has been shown to independently predict incident cardiovascular disease (CVD) in subjects with type 2 diabetes.10
A study of patients with type 2 diabetes found that as patients move towards their glycaemic target, the relative importance of targeting FPG versus PPG changes.11 FPG levels have a greater impact in those with poor glycaemic control whereas PPG levels make a greater contribution to HbA1c levels in patients with better glycaemic control. For example, at HbA1c levels of <7.3 % PPG contribute around 70 % of the HbA1c. However, at HbA1c levels exceeding 10.2 %, FPG contributes 70 % of the value, and PPG contributes the remaining 30 %. Worsening diabetes control is preceded by changes in daytime post-prandial control, followed by changes during the morning, and finally by changes in nocturnal fasting control.12 These findings may explain the limited ability of patients to achieve HbA1c goals even when
FPG levels appear to be controlled and the fact that in treat-to-target trials, decreases in FPG levels were not accompanied by target reductions in HbA1c.13,14 Several studies have shown that improvement of post-prandial hyperglycaemia is associated with reductions of both FPG and HbA1c.15 One study found that, when PPG goals (<140 mg/dl) were achieved, 94 % of patients reached the HbA1c goal of <7 % compared with only 64 % when FPG goals (<100 mg/dl) were attained and concluded that control of post-prandial hyperglycaemia is essential for achieving recommended HbA1c goals.16 Thus, a reasonable recommendation for PPG testing and targets is that for patients who present FPG values within target but have HbA1c values above target, monitoring PPG 1 to 2 hours post-meal and treatment aimed at reducing PPG values to <180 mg/dl may help lower HbA1c.17
As mentioned above, evidence of a strong correlation between high PPG levels and the development of vascular complications underscores the significance of targeting PPG. PPG has been associated with markers of atherosclerosis, inflammation, endothelial dysfunction and oxidative stress,18,19 as well as other complications including retinopathy,20 increased cancer risk21,22 and impaired cognitive function in elderly patients.23 Poor control of PPG has been associated with an elevated risk of CVD,24,25 particularly in women.26 The association between PPG elevation and cardiovascular morbidity and mortality is independent of FPG.27 Therapy targeted at PPG control has been shown to reduce the progression of atherosclerosis and CV events.28 Therefore, attaining glycaemic control, and reducing CVD burden associated with type 2 diabetes, may be difficult without adequate control of PPG levels.
There is a need to implement intensive glycaemic control as early as possible in the progression of type 2 diabetes to prevent the development of microvascular and cardiovascular complications.24,29 Basal insulin therapy achieves good glycaemic control in 50–60 % of patients.30 However, a significant proportion of patients treated with basal insulin therapy who have elevated HbA1c may experience inadequate control of PPG. Traditionally, dual targeting has been achieved with insulin therapy using a basal insulin to target FPG and a prandial insulin for PPG control, either as part of a basal-bolus or biphasic insulin regimen.31 However, this approach has limitations, including greater complexity for patients, increased risk of hypoglycaemia and likelihood of leading to weight gain.32 Thus, it could be considered that adequate control of PPG remains an unmet clinical need in diabetes therapy.
Glucagon-like Peptide 1 Receptor Agonists
Mechanisms responsible for hyperglycaemia in type 2 diabetes include not only a decline in beta-cell function and insulin resistance, but also increased levels of glucagon, resulting in increased production of hepatic glucose and therefore elevated FPG and PPG. It is also known that ingested glucose causes a greater insulin response than glucose administered intravenously, a result of the release of gastrointestinal hormones – the incretin effect.33 Glucagon-like peptide (GLP-1) is a naturally occurring incretin hormone that is released by the L-cells located in the gastrointestinal tract within minutes of ingesting glucose. Importantly, GLP-1 counters the effects mechanisms responsible for hyperglycaemia in type 2 diabetes by suppressing glucagon secretion from pancreatic alpha cells as well as stimulating insulin secretion by beta cells.34–36
Targeting the incretin system has become an important therapeutic approach in type 2 diabetes. In addition to their glucose-lowering effects, GLP-1 receptor agonists possess a number of beneficial clinical characteristics. GLP-1 receptor agonists do not only stimulate insulin secretion and inhibit glucagon output in a glucose-dependent manner, but also slow gastric emptying and decrease appetite. As a result, GLP-1 receptor agonist therapy in type 2 diabetes results not only in improved glycaemic control with low rates of hypoglycaemia, but also in either weight loss or suppression of weight gain.37,38 GLP-1 receptor agonists have been associated with improved levels of PPG, but target both FPG and PPG. Furthermore, GLP-1 receptor agonists also have the potential to preserve pancreatic beta cells, which may provide long-term metabolic control.39
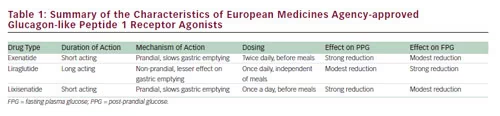
Three GLP-1 receptor agonists are currently approved in Europe for use in type 2 diabetes. Other GLP-1 receptor agonists (albiglutide, dulaglutide and semaglutide) are in clinical development. The first commercially available GLP-1 agonist, exenatide (Byetta®, Amylin Pharmaceuticals) was approved by the European Medicines Agency (EMA) in 2006 and is administered twice daily as a subcutaneous injection.40,41 In some European countries, exenatide is also available as an extended-release formulation, which is given as a once-weekly injection.42 Liraglutide, administered once daily, (Victoza®, Novo Nordisk) received EMA approval in 2009 and provides improved glycaemic control compared with exenatide.43,44
Lixisenatide (Lyxumia®, Sanofi) is a once-daily prandial GLP-1 receptor agonist for the treatment of type 2 diabetes. Lixisenatide is a 44-amino acid peptide that is amidated at the C-terminal end and shares some structural elements with exendin-4, the main difference being the addition of six lysine residues at the C-terminus.45 Lixisenatide is short acting but given as a once-daily dose.46 A number of studies have demonstrated that lixisenatide affects numerous factors involved in glucose regulation.47–50 In 2013, the use of lixisenatide was approved by the EMA for the treatment of type 2 diabetes to achieve glycaemic control in combination with oral glucose-lowering products and/or basal insulin when these, together with diet and exercise, do not provide adequate glycaemic control, and it has been submitted for US Food and Drug Administration (FDA) approval.
GLP-1 receptor agonists provide significant improvements in glycaemic control. However, they have widely differing pharmacokinetic and
pharmacodynamic profiles.51,52 GLP-1 receptor agonists have been categorised as either short- or long-acting compounds. Short-acting GLP-1 receptor agonists, such as exenatide and lixisenatide, activate the GLP-1 receptor for only around 6 hours after each injection.46,53 The recommended dosing intervals are twice daily for exenatide (before breakfast and dinner) and once daily for lixisenatide (usually before breakfast).51 Long-acting compounds (such as liraglutide, albiglutide, dulaglutide and exenatide extended release [ER]), require only daily dosing (see Table 1).
In order to optimise the use of GLP-1 receptor agonists, an understanding of their differing mechanisms of action is necessary.54 While all GLP-1 receptor agonists share the same fundamental mechanism of action on the GLP-1 receptor, they also exhibit important differences. Lixisenatide and exenatide primarily exert their main effects during the prandial period. Their effects on PPG are not mediated by stimulation of insulin secretion; in fact, they reduce post-prandial insulin secretion. Their effect on PPG is a result of delayed gastric emptying, which decreases the rate of entry of glucose into the duodenum and subsequently into the circulation.55 This concept has been verified using native GLP-1.56 Longeracting GLP-1 agonists are subject to tachyphylaxis for their initial effect to slow gastric emptying owing to their sustained receptor activation, whereas short-acting agent have a sustained and substantial effect.
The effect of lixisenatide on gastric emptying was confirmed in a recent study. Patients were randomised to lixisenatide (20 μg daily) and placebo, respectively. In the lixisenatide group, a reduction in PPG was seen when compared with placebo throughout the day: after breakfast (p<0.0001), lunch (p<0.0001) and dinner (p<0.05). Gastric emptying (50 % emptying time) increased substantially from baseline with lixisenatide, but not with placebo (change from baseline ± SD: –24.1 ± 133.1 minutes for placebo and 211.5 ± 278.5 minutes for lixisenatide; p<0.01).50 Despite its relatively short half-life, morning administration of lixisenatide exhibited a pharmacodynamic effect on blood glucose throughout the day. Lixisenatide is therefore suitable for once-daily dosing.
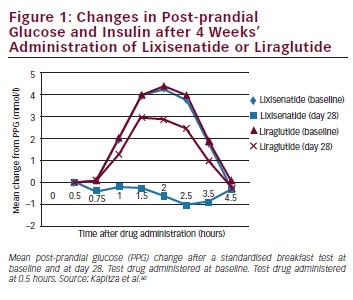
Long-lasting GLP-1 receptor agonists such as liraglutide and exenatide ER can be administered at any time of day and show elevations in plasma levels of the drug throughout the period between doses. However, their mode of action differs from the short-acting, prandial agents. While it results in a more continuous activation of the GLP-1 receptor, the effect of liraglutide on gastric emptying is short lived.57 This is probably a result of tachyphylaxis, meaning that the effect on gastric emptying decreases rapidly with time, owing to continuous activation of the GLP-1 receptor. A comparison of twice-daily exenatide and exenatide ER taken once weekly, showed that the former had the greater effect on gastric emptying.54 Liraglutide has a stronger effect on FPG, which is mediated by its effect on beta cell function.58,59 As a result of these differing mechanisms of action, GLP-1 receptor agonists have differing effects on PPG and FPG. A comparative study of liraglutide and exenatide found that liraglutide had a greater impact on FPG levels, while exenatide primarily affected PPG.43,60 Lixisenatide has demonstrated particular efficacy in lowering PPG.50 A comparative study found that lixisenatide provided a significantly greater reduction of PPG compared with liraglutide during a standardised solid meal test (see Figure 1).60 The properties of the three EMA-approved GLP-1 receptor agonists are summarised in Table 1.
Antibody formation has been reported in patients treated with GLP-1 receptor agonists.61 It is possible that exenatide and lixisenatide may result in greater antibody formation, possibly because of a lower homology with native GLP-1. However, there is a lack of antibody data for lixisenatide, and the relevance of antibody formation remains unclear.
Combined Use of Basal Insulin with Glucagon-like Peptide 1 Receptor Agonists
Because of their complementary mechanisms of action, GLP-1 agonists are being increasingly used in combination with basal insulin analogues. Basal insulin therapy primarily targets FPG, and insulin-based strategies targeting PPG including the addition of rapid acting insulin to basal insulin have been associated with hypoglycaemia and weight gain.31 On the other hand, GLP-1 receptor agonists may target FPG or PPG and their use is associated with low rates of hypoglycaemia and modest weight loss. Clinical data have demonstrated the effectiveness of the combined basal insulin and GLP-1 agonist regimens.62–66 Of interest, the addition of a prandial GLP-1 receptor agonist (targeting PPG) with basal insulin (targeting FPG) offers a reduced risk of hypoglycaemia compared with combined insulin regimes and may offer a new treatment paradigm for type 2 diabetes. Future studies should formally compare the efficacy of basal plus prandial insulin regimes with combined regimens involving basal insulin and GLP-1 receptor agonists.
Lixisenatide Clinical Trial Data
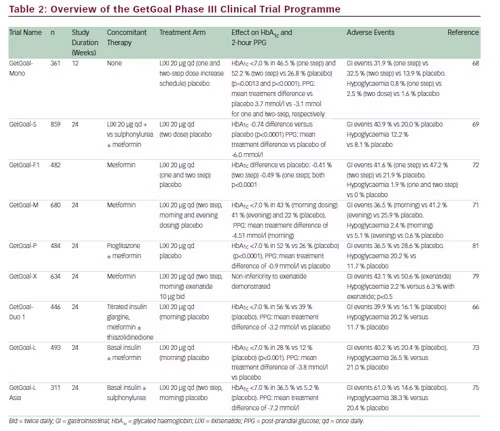
A large body of clinical trial data has been amassed from the GetGoal clinical development programme. More than 5,000 patients with type 2 diabetes were enrolled in GetGoal globally and it comprised 11 phase III trials that assessed the efficacy and safety of lixisenatide in monotherapy, as add-on therapy to metformin, sulphonylureas or thiazolidinediones, in combination with basal insulin and a comparative trial with exenatide.52,67 Results of these trials are summarised in Table 2. In all studies, lixisenatide resulted in significant improvements in HbA1c and PPG, as well as modest beneficial effect on body weight, compared with placebo.
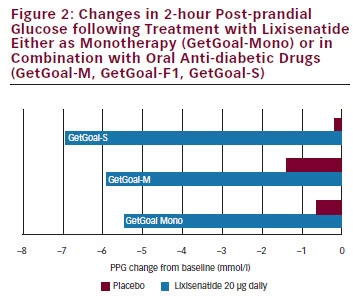
In GetGoal-Mono, which assessed the efficacy and safety of lixisenatide as monotherapy, lixisenatide had a marked impact on PPG, particularly after breakfast, with substantial reductions in 2-hour PPG excursion compared with placebo (see Figure 2).68 This study also established that only small differences in HbA1c reduction were seen when lixisenatide was administered as one-step dose increase (10 μg for 2 weeks, then 20 μg) and a two-step dose increase (10 μg for 1 week, 15 μg for 1 week, then 20 μg).
Lixisenatide has also demonstrated efficacy as add-on therapy to oral anti-diabetic drugs (OADs). GetGoal-S evaluated the efficacy and safety
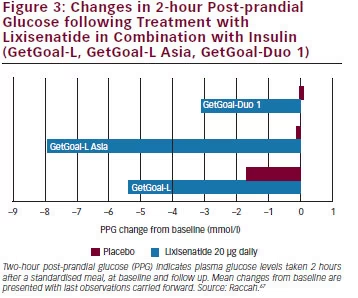
of lixisenatide in patients insufficiently controlled on sulphonylurea and/ or metformin (see Figure 2).69 As an add-on therapy to sulphonylurea with or without metformin, lixisenatide significantly reduced HbA1c levels and improved 2-hour PPG and FPG levels. GetGoal-P demonstrated the efficacy of lixisenatide as an add-on therapy to pioglitazone with or without metformin.70 GetGoal-M found that, as add-on therapy to metformin, lixisenatide, given as a morning or evening dose, significantly improved glycaemic control in patients whose diabetes was inadequately controlled with metformin.71 GetGoal-F further established the efficacy of lixisenatide as add-on therapy to metformin and investigated a one-step dose increase versus a two-step dose increase. The study found that a one-step dose increase regimen may be the best option for treatment initiation.72 The GetGoal clinical trial programme also included a comprehensive assessment of the combined use of basal insulin and lixisenatide (GetGoal-L, GetGoal-Duo-1 and GetGoal L-Asia) and demonstrated significant benefits in patients that do not achieve glycaemic control on basal insulin analogues alone (see Figure 3) either in early insulinised patients or in those treated with a stable insulin regimen. In GetGoal-Duo-1, insulin glargine was titrated in a 12-week run-in phase. Patients whose HbA1c was >7 % were randomly assigned to once-daily lixisenatide at or placebo for 24 weeks while insulin glargine titration continued.66 In GetGoal L, patients with longstanding type 2 diabetes and inadequate metabolic control (HbA1c 8.4 %) while on basal insulin therapy were randomised to the addition of lixisenatide 20 μg once daily or placebo for 24 weeks.73 GetGoal L-Asia included a study of lixisenatide as an add-on to basal insulin in Asian patients with type 2 diabetes. Asian patients often have insulin deficiency rather than insulin resistance and impaired secretion of GLP-1.74 GetGoal-L-Asia found that the addition of lixisenatide as add on to basal insulin therapy improved HbA1c and PPG and demonstrated the safety and efficacy of lixisenatide with or without a sulphonylurea.75
In all trials, lixisenatide significantly decreased mean HbA1c and improved PPG.66,71,73,76–78 In all the completed studies, lixisenatide was generally well tolerated: as monotherapy treatment-related adverse effects (AEs) were similar to placebo (53.6 % versus 45.1 %). The most common AEs were gastrointestinal (32.5 % versus 13.9 % placebo), with nausea being the most frequent (22.2 % versus 4.1 %), although the majority of these AEs were rated mild to moderate and resolved without the need for treatment.68 When administered as monotherapy or as an add-on to OAD therapy, lixisenatide did not increase the frequency of hypoglycaemia compared with placebo and was associated with very low rates of severe hypoglycaemia. In GetGoal-F1 and GetGoal-S, lixisenatide was associated with weight reductions of approximately 1 kg over the 24- week study periods.69,72 The use of lixisenatide has also been associated with improved gastrointestinal tolerability, with fewer gastrointestinal events (especially nausea, 24.5 versus 35.1 %; p<0.05) and lower incidence of symptomatic hypoglycaemia compared with exenatide (2.5 versus 7.9 %; p<0.05, GetGoal X).79 The safety and tolerability of lixisenatide was also shown to be consistent across all age groups.78 In addition to the GetGoal programme, a recent trial comparing lixisenatide with liraglutide found that pre-breakfast lixisenatide showed a significantly greater reduction in PPG during a morning test meal versus pre-breakfast liraglutide, and showed significant decreases in post-prandial insulin, C-peptide (versus an increase with liraglutide) and glucagon, and better gastrointestinal tolerability than liraglutide (see Figure 1).60
As well as providing data demonstrating its efficacy and safety on glycaemic control, the clinical development programme of lixisenatide also includes a CV outcomes trial. The effects of lixisenatide on cardiovascular outcomes in patients with type 2 diabetes who have recently experienced a cardiac event are being evaluated in 6,000 patients in the 44-month Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE0010 (Lixisenatide) (ELIXA) trial,80 with estimated completion of the study in 2014.
Summary and Concluding Remarks
Although the treatment of type 2 diabetes has focused on the control of FPG and HbA1c, recent findings suggest that FPG does not always correlate well with HbA1c and that PPG plays a greater role in glycaemic control. The development of GLP-1 receptor agonists has increased the treatment options for patients with type 2 diabetes, offering improved glycaemic control with a reduced risk of hypoglycaemia compared with other treatment options. The currently approved GLP-1 receptor agonists differ both in their duration and mechanism of action. Lixisenatide offers advantages over both liraglutide and exenatide respectively in that it is relatively short acting and effectively targets PPG and only requires daily dosing. Data from the GetGoal Phase III programme has demonstrated that treatment with lixisenatide optimises glycaemic management by controlling PPG. Daily treatment with lixisenatide provides a simple, convenient treatment option for patients with type 2 diabetes, particularly in patients for whom the risk of hypoglycaemia is a concern.
The pronounced effect of lixisenatide on PPG provides a strong rationale for combining it with long-acting basal insulin analogues, in cases where the latter is not providing adequate glycaemic control. Patients who have met their FPG target but not their HbA1c goals require prandial therapy to fill this unmet need. Lixisenatide, as add-on therapy to basal insulin therapy, can help meet this need. Its additive effects on glycaemic control combined with a potential benefit on beta cells, beneficial effect on body weight and limited additional risk of hypoglycaemia may lead to a new treatment approach to manage blood glucose and prevent long-term complications in patients with type 2 diabetes.







