Pituitary tumors are the most common form of intracranial neoplasms.
Their prevalence in autopsy series was reported as 5–20 %.1
However,
clinically relevant pituitary tumors presenting with disturbances of
hormonal secretion or mass effect are rare, with an estimated prevalence of
200/1,000,000 and an incidence of 2/100,000 per year.2
Therefore, they only
represent about 10 % of all surgically resected intracranial neoplasms.3,4
Their prevalence increases with advancing age: both sexes are affected
equally. They can cause a variety of endocrine syndromes and disorders1
including panhypopituitarism, acromegaly, Cushing’s disease, infertility,
and visual disturbances. Recently, the diagnosis of pituitary adenomas
has increased as a result of the advances in neuroimaging technologies.
Due to endocrine hyperfunction, hormonally active adenomas are usually
diagnosed at an earlier stage than hormonally inactive ones, which are
mostly diagnosed due to the effect of local pressure exerted by the
growing tumor.5
Based on their size, pituitary adenomas can be divided into microadenomas
and macroadenomas, the latter being reserved for adenomas larger than
10 mm in diameter. On the other hand, the first histologic classification of
pituitary tumors was based on tinctorial characteristics using hematoxylineosin
stains on resected tissue. Tumors were accordingly classified as
eosinophilic, basophilic, or chromophobic adenomas4
and were suggested
to be associated with acromegaly, Cushing’s syndrome and nonfunctioning
adenomas, respectively. Later studies made such a classification irrelevant
by showing that some acidophilic tumors do not produce growth hormone
(GH) and some GH-producing tumors are not acidophilic. Similarly, some
basophilic tumors do not cause Cushing’s syndrome and more than half
of the chromophobic tumors are endocrinologically active, secreting
various hormones.4
Fortunately, with the development of immunohistochemistry (IHC),
correlation of clinical features and endocrine activity became possible. IHC
studies permit a conclusive identification of the various cell types in the
pituitary adenomas. Currently, the standard immunohistochemical battery
includes the use of antibodies to GH, prolactin (PRL), adrenocorticotrophic
hormone (ACTH), thyroid-stimulating hormone (TSH), follicle-stimulating
hormone (FSH), luteinizing hormone (LH), and the a-subunit of the
glycoprotein hormones.4
Generally these hormones are expressed
either singly or in various combinations. Although cellular hormonal
immunoreactivity is common in pituitary tumors, there are many exceptions
where the immunocytochemical staining is not concordant with the clinical
or biochemical features. Classic examples are the silent corticotroph and
somatotroph adenomas, in which tumor cells stain positively for ACTH
and GH, respectively, and yet patients have no clinical or biochemical
features of excessive hormone secretion.1,6–9 Silent or nonfunctioning
adenomas have no clinical expression of produced hormones, either
because they produce inactive molecules or do not release sufficient
amount of hormones from cells to create a detectable blood level.4
Prolactinomas represent the majority of clinically recognized pituitary
adenomas, accounting for approximately 40–45 % of all.3
However, their
incidence among reported surgical series is lower because of the medical
therapeutic option of these tumors.3
They are reported to occur more
frequently in women than in men, particularly between the second and third
decades of life, when the ratio is estimated to be 10:1. Prolactinomas vary
in size at presentation with most women presenting with microadenomas,
whereas men tend to have macroadenomas at diagnosis. In women,
hyperprolactinemia causes oligomenorrhea or amenorrhea as well as
galactorrhea. In men, however, the main presenting symptom is impotence
and diminished libido, which can often be overlooked and attributed to
other causes.1
Generally, Immunohistochemical study of prolactinomas, show a high
rate of PRL expression in concordance with serum PRL levels. They grow
slowly with Ki-67 staining of less than 2 %.8
Here, we report an interesting
case of pituitary adenoma with high serum levels of PRL and histologically
confounding sparse PRL expression. The patient manifested a longstanding
history of amenorrhea and presented with abrupt apoplectic
hemiplegia due to a thalamic infarction resulting from carotid compression
by the tumor.
Case Report
A 25-year-old Middle Eastern female was brought to the outpatient clinic
with complaints of right-sided hemiparesis, mild right-sided facial paralysis,
aphasia, and a long-standing history of amenorrhea. Her menstrual cycles
became irregular 8 years ago and then completely stopped to occur. Her
family reported that her hemiparesis started in her right hand 2 weeks ago
and progressed into a fully blown hemiplegia within 2 days. On admission,
she seemed lethargic and was only able to speak with incomprehensible
words, partially able to follow commands on her left side. The visual tests
were unable to be performed due to lack of adequate cooperation of the
patient. Hyperactive deep tendon reflexes and diminished muscle tone
were noted on her right-sided extremities.
Magnetic resonance (MR) imaging of the head showed a lobulated,
encapsulated mass lesion, occupying the sella turcica, causing a notable
expansion of the sella, extending into the suprasellar cistern and invading
both cavernous sinuses (see Figure 1-A, D). Upward displacement of the
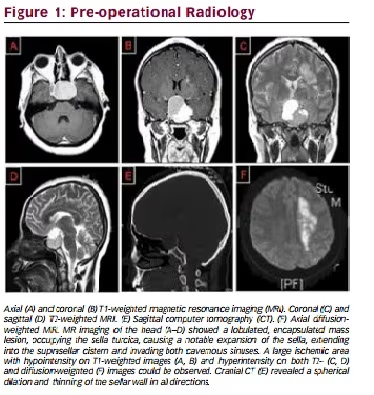

optic chiasm and hypothalamus was noted. Also, the left carotid syphon
seemed to have a remarkably diminished caliber compared with the right.
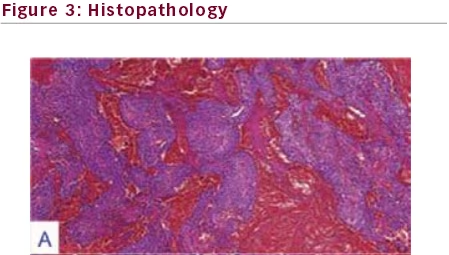
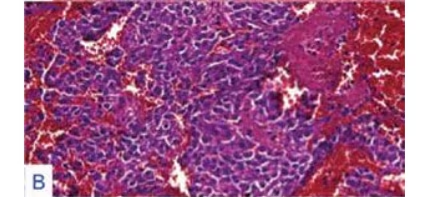
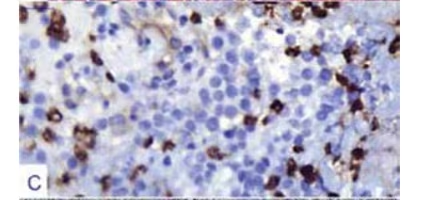
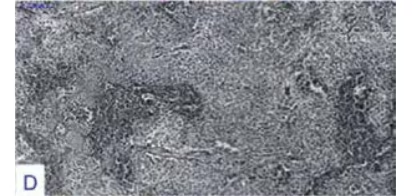
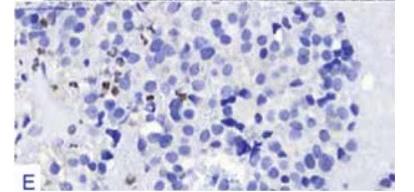
Original magnification factors and staining techniques are given in parenthesis. A. Pituitary
adenoma with extensive hemorrhagic foci were displayed (hematoxylin-eosin [H&E]
staining, x100). B. Monotonous adenoma cells displayed hyperchromatic nuclei wrapped by
acidophilic cytoplasm with intervening extravasated erythrocytes (H&E staining, x200). C.
Adenoma cells exhibited scarce prolactin [PRL] immunoreactivity (immunohistochemistry
[IHC] with streptavidin biotinylated complex [SBC] and PRL antibody, x400). D. Adenoma
was shown to lack reticulum framework, which is assumed to be a striking feature of
pituitary neoplasms (silver impregnating staining, x40). E. Adenoma cells exhibited a low
Ki67 labeling index of <1 % (IHC with SBC and Ki67 antibody, x400).
The sellar lesion showed a cystic expansion to the right and superior
aspect, while revealing a solid component with mottled enhancement on
the left after gadolinium injection. Left carotid syphon was noted to be
under compression due to a pronounced invasion of the left cavernous
sinus by the lesion. Also, a large ischemic area with hypointensity on
T1-weighted images (see Figure 1-A, B) and hyperintensity on both T2-
(see Figure 1-C, D) and diffusion-weighted (see Figure 1-F) images was
noted. Commensurate with the patient’s hemiparesis, the ischemic region
involved the left thalamic structures and the left internal capsule as well as
subcortical sections of the pyramidal tract. Slight contrast enhancement
and hypointense impression on ADC map of the ischemic lesion was
in agreement with a subacute infarction coinciding with the history of
stroke happening 2 weeks earlier. A cranial computed tomography (CT)
was ordered to investigate for bony erosion, which revealed a spherical
dilation and thinning of the sellar wall in all directions (see Figure 1-E).
No signs of complete erosion or cerebrospinal fluid (CSF) fistula were
found. Pituitary adenoma, craniopharyngioma, and meningioma were
considered among differential diagnoses. Cardiac workup including an
echocardiography was performed to rule out any possible cardiogenic
etiology of thromboembolism, which returned normal results.
Complete blood count (CBC) and biochemical workup revealed normal
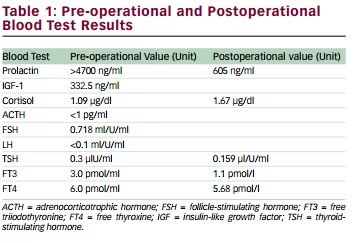
results including normal electrolytes. Blood tests results for pre-op levels
of PRL, insulin-like growth factor 1 (IGF-1), ACTH, cortisol, FSH, and LH are
given in Table 1. All pituitary hormonal axes were undisturbed except for
PRL and ACTH. Prolactin level was measured at 4,700 ng/ml. The patient
was considered to have a prolactinoma. Immediate surgical treatment was
planned and a left pterional craniotomy was performed. Sylvian dissection
was used to expose the suprasellar mass. The tumor cyst was easily
accessible through optico-carotid angle due to sellar expansion. Left carotid
artery seemed pale due to tumor compression. A soft, pink-to-purple cyst
wall was noted. The cyst was incised and internal decompression was
obtained. The cyst was filled with a necrotic yellow-colored paste, which
was attributed to the apoplectic bleeding thought to occur 2 weeks earlier.
Pink-to-purple chunks of tumor tissue were evacuated from the bottom of
the encapsulating cyst, by using pituitary ring curettes. Intraoperative frozen
section study of these chunks strongly indicated a pituitary adenoma. The
remaining cystic wall was dissected away from both optic nerves and
the right carotid artery. Normal pituitary gland and a thinned stalk were
noted on the right side of the sella adherent to tumor capsule. However,
the capsule was strictly adhered to the left carotid artery and the bottom
of the sella. Therefore, the adhered capsule was left in place in order to
avoid carotid injury. Following closure, the patient was not extubated and
moved to an intensive care unit (ICU) under continuing sedation in order
to prevent cerebral revascularization injury. She was extubated the next
day and immediate improvement in her hemiparesis was noted. Postop
MRI revealed a residual tumor in the left cavernous sinus (see Figure
2). Prolactin level was decreased to 605 ng/ml on postop day 1 and
she was immediately started on cabergoline. Also, hypothyroidism and
hypocortisolemia were noted, which were replaced by levothyroxine and
methylprednisolone, respectively. She also developed a transient diabetes
insipidus, which spontaneously resolved within 4 days. She was moved out
of ICU on postop day 2 and started on physiotherapy sessions.
Histologic examination of the tumor under light microscopy revealed
an acidophilic adenoma (see Figure 3-B) with hyperchromatic eccentric nuclei surrounded by polygonal, abundant cytoplasm. In addition to
widespread minute hemorrhages (see Figure 3-A), apoptotic bodies
were extensively scattered in tumor tissue, which were attributed to the
apoplectic bleeding. No evidence of multinucleate cells or mitotic figures
was present. Adenoma tissue was lacking a reticulin framework with silver
impregnation stain (see Figure 3-D). Immunohistochemical battery of
pituitary endocrine markers, including GH, ACTH, LH/FSH, and TSH were all
negative and surprisingly, there was only a scarce PRL immunoreactivity
that was less than 20 % (see Figure 3-C). Methylated O6-methylguanineDNA
methyltransferase (MGMT) reactivity was 10–20 %. Nuclear p53
oncoprotein reactivity was negative. A low Ki67 LI of <1 % was calculated
(see Figure 3-E). No immunoreactivity for pancytokeratin was present.
These immunohistopathologic features were consistent with a sparsely
granulated PRL cell adenoma undergoing an extensive pituitary apoplexy.
Therefore, the final diagnosis of sparsely granulated PRL cell adenoma was
reported despite very high serum PRL levels.
After the patient was released, cabergoline treatment was continued.
Blood PRL levels were monitored regularly and the values at both month 4
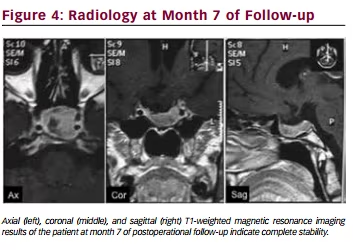
and 7 were 50 ng/ml. MR imaging at 7 months after the operation indicated
complete stability (see Figure 4). She reported a complete recovery of her
motor functions.
Discussion
Prolactinomas are slow-growing tumors and constitute the main group
of patients with pituitary adenoma. They present with serum PRL levels
exceeding 200 ng/ml and histologically show strong PRL immunoreactivity.
However, the histologic examination of the adenoma in our case revealed
sparse PRL immunoreactivity despite exceedingly high PRL secretion
with a serum level at 4,700 ng/ml. Prolactinomas, typically, show a good
correlation between tumor volume and serum PRL level with 100 % PRL
immunoreactivity of tumor cells. However, pituitary adenomas are known
to present with hormone expression patterns incongruous with detectable
serum hormone levels. Ogawa et al. reported on a case of null cell adenoma
with weak somatostatin expression and low serum hormone levels.9
Nonetheless, we could not find any reports of prolactinomas with sparse
PRL expression associated with extreme serum PRL levels. Kovacs et al.
proposed that either fully or scarcely, PRL immunoreactive cases might
express a specific ultrastructural pattern consistent with lactotropes, such
as a rough-surfaced endoplasmic reticulum with Nebenkern formation,
prominence of Golgi apparatus, presence of misplaced exocytosis, as well
as pleomorphism of secretory granules with a considerable variation of
size ranging from 130 to 500 nm in diameter.10 They also suggested that
periadenomatous lactotropes might be oversecreting PRL in a null cell
adenoma, which might have been given rise to hyperprolactinemia.10
In our case, the patient was diagnosed with prolactinoma considering the
combined results from MR and CT imaging and high serum PRL levels. While
the radiologic tests revealed the size, structure, and location of the tumor,
the blood tests confirmed the differential diagnosis as a prolactinoma.
Astoundingly, the immunohistopathologic features revealed a few cells with
PRL immunoreactivity and no other hormone expression. Therefore, the
patient was considered to have a sparsely granulated PRL cell adenoma. For
PRL-secreting pituitary adenomas (or prolactinomas), the size of the tumor
usually determines the magnitude of the PRL secretion.11,12 For instance, PRL
serum levels higher than 200 ng/ml is often accepted as the specific cut-off
value for prolactinoma, whereas this value is usually less than 100 ng/ml for
the stalk effect caused by other types of parasellar tumors by preventing
inhibitory control of dopamine secreted from hypothalamus.9
An extreme increase in serum PRL concentrations suggests a prolactinoma.
However, with giant prolactinomas, as in our case, radioimmunoassay
measurements of serum PRL concentrations might result in falsely low
values because of the ‘hook’ effect phenomenon.13 The initial serum PRL
levels in such cases may be only mildly increased (50–150 g/l). However,
the actual concentrations are often measured to be in range of several
thousands by the serial dilution method. Therefore, in patients with
giant tumors (>3 cm), if the initial serum PRL concentration is moderate,
serial dilutions must be performed. This process is clinically important to
differentiate patients with prolactinomas from others.1
Treatment of apoplectic pituitary tumors is generally surgical intervention.
The apoplectic bleeding in our patient resulted in a devastating loss
of neurologic functions. Therefore, immediate surgical treatment was
performed. However, dopamine agonist therapy is the primary treatment
for most prolactin-secreting adenomas, even in patients with suprasellar
extension and chiasmal compression.8,11 This treatment effectively
normalizes the serum PRL concentration in approximately 85 % and shrinks
the size of the tumor in approximately 70 % of the patients. Regular followups
and careful monitoring of the patients are critical to ensure tumor
regression in response to medical therapy.1
In cases resistant to medical
treatment, surgical removal via transsphenoidal or transcranial routes
can be considered in the first 3 or 4 months.14 Surgical approach depends
to a large degree on tumor size, its extent, the patient’s preference, and
the available expertise for the managing surgeon. It should be noted that
tumor extensions beyond the sella decreases the success probability of the
surgery to less than 50 %.14 However, experienced surgeons may achieve
complete or near-complete adenomectomy, with normalization of serum
PRL concentration in 60–70 % of the patients. In 20–50 % of these patients,
recurrences may occur over a 10-year period.11–14
Another option for treatment of adenomas is radiotherapy. Traditional
radiotherapy is largely being replaced by gamma knife radiosurgery lately
due to its increasing availability. Gamma knife treatment is reserved for
patients with microadenomas resistant to medical treatment or with
residual tumors following surgery.15 Although it is well known that the brain tumors may cause stroke, pituitary
adenoma accompanied with ischemia and stroke is rare.16–18 In our case,
MRI findings showed that the reason for the patient’s hemiplegia was
the ischemia in the left thalamic structures and internal capsule as well
as subcortical sections of the pyramidal tract. Most of the symptomatic
obstructions of internal carotid arteries in pituitary adenoma patients are
reported to result from pituitary apoplexies. Pituitary apoplexy, a severe
yet uncommon complication of pituitary adenomas, is characterized as
enlargement of the tumor due to sudden hemorrhage or ischemia.16 In
our case, carotid compression was noted intraoperatively by the pale
coloration in respect to the opposite side. The most likely explanation of a
stroke in pituitary apoplexies is the extraluminal compression of the main
brain vessels caused by the acute growth of the tumor. Elevated intrasellar
pressure in the first weeks of pituitary apoplexy might lead to vessel
occlusion.19 Especially in giant tumors, the development of collateral
vessels indicates chronically compromised blood flow.17 Also, the use of
dopaminergic agonists has been suggested to pose an increased risk for
pituitary apoplexy.16 In most cases, a CT scan is adequate to demonstrate
a pituitary apoplexy, which mandates surgical removal of the tumor. Early
surgical decompression restores the blood flow and facilitates recovery of
neurologic symptoms. In our case, hemiplegia was improved immediately
after surgical removal of the tumor.