Growth hormone deficiency (GHD) causes low growth velocity in childhood, and if left untreated, severe short stature in adulthood. It is diagnosed by examining auxological data, thorough clinical examination and provocative testing. Patients with congenital GHD may have other pituitary hormone abnormalities and abnormal pituitary anatomy.
Growth hormone deficiency (GHD) causes low growth velocity in childhood, and if left untreated, severe short stature in adulthood. It is diagnosed by examining auxological data, thorough clinical examination and provocative testing. Patients with congenital GHD may have other pituitary hormone abnormalities and abnormal pituitary anatomy.
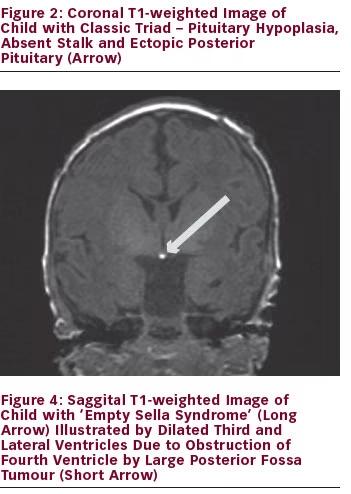
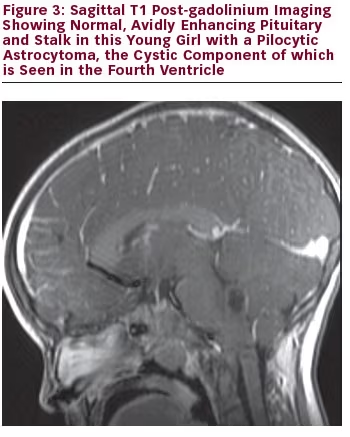
Magnetic resonance imaging (MRI) is the best tool in delineating pituitary anatomy and pathology. On MRI, patients with congenital hypopituitarism may have the ‘classic triad’ of pituitary stalk interruption syndrome, which consists of: (a) an interrupted or thin pituitary stalk; (b) an absent or ectopic posterior pituitary (EPP); and (c) anterior pituitary hypoplasia or aplasia 1 see Figures 1–3 for examples. Approximately 50% of patients with idiopathic GHD have been shown to have abnormal pituitary anatomy on MRI.2,3 MRI is an important marker for the anticipation of future endocrine dysfunction, as patients with abnormal pituitary anatomy are more likely to have multiple endocrinopathies.3
The Pituitary Gland – A Physiological Overview
The hypothalamus and the pituitary gland function as a ‘control centre’ for several endocrine glands, including the thyroid, adrenals and gonads.
The human foetal anterior pituitary is recognisable at four to five weeks gestation, and the hypothalamic–pituitary unit is mature at 20 weeks.4 The anterior pituitary (also known as the adenohypophysis) originates from Rathke’s pouch, which is an extension of the oral ectoderm. The Rathke’s pouch moves up from the oral cavity and comes in contact with the posterior pituitary (also known as the neurohypophysis). The posterior pituitary arises from an evagination of the ventral hypothalamus and the third ventricle. Remnants of Rathke’s pouch may persist and lead to colloid cysts or craniopharyngioma.4,5
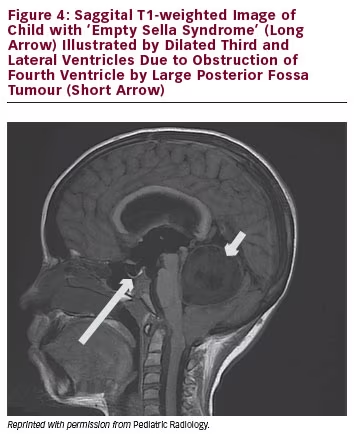
The pituitary gland is encased in the sphenoid bone at the base of the skull in the sella turcica (‘Turkish saddle’). The diaphragma sellae prevents the cerebrospinal fluid (CSF) from entering the sella turcica. If the diaphragma sellae does not form properly, this can allow CSF to enter the sella turcica and compress the pituitary, giving the appearance of ‘empty sella syndrome’.5 When this occurs, the pituitary tissues do not retain a normal appearance and the function of the gland may or may not be affected. CSF rhinorrhea or visual field abnormalities have been described in patients with ‘empty sella syndrome’. See Figure 4 for example of empty sella syndrome secondary to intracranial mass.
The anterior pituitary gland has a very rich blood supply. Arterial blood is supplied from the internal carotid arteries via the superior, middle and inferior hypohysial arteries. The pituitary stalk and the posterior pituitary are supplied directly from branches of the middle and inferior hypophysial arteries.4
Anterior pituitary cells are classified into four cell types based on their secretory products. Somatotrophs secrete GH and account for 50 % of adenohypophysial cells.4 GH stimulates production of insulin-like growth factor (IGF-1) and directly affects growth and metabolism. Lactotrophs account for 10–25 % of anterior pituitary cells and they secrete prolactin, which stimulates milk production in lactation.4 Thyrotrophs secrete thyroid stimulating hormone/thyrotropin (TSH) and account for 10 % of anterior pituitary cells.4 TSH stimulates thyroid gland function, including hormone synthesis, secretion, hyperplasia, hypertrophy and vascularisation. ACTH and related peptides are secreted by corticotrophs, which make up 15–20 % of the cells in this gland.4 This hormone stimulates the production of glucocorticoids and weak androgens in the adrenal gland. Lastly, gonadotrophs, which account for 10–15 % of the cells in the anterior pituitary,4 secrete luteinising hormone (LH) and follicle stimulating hormone (FSH). LH stimulates steroid hormone synthesis in the ovarian theca interna cells, lutein cells and hilar cells or the Leydig cells in the testes. FSH promotes follicle development in the ovary and promotes spermatogenesis in the sertoli cells of the testes. Patients with anatomical abnormalities of the pituitary gland may have dysfunction in none, one, several or all of these hormones.
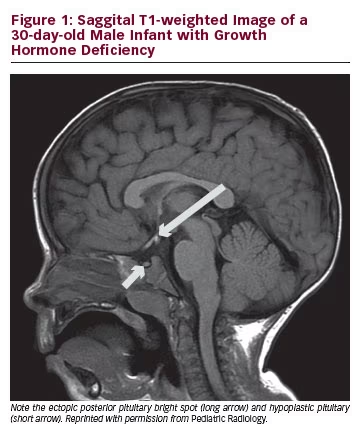
The neurohyophysis secretes vasopressin (also known as ADH or antidiuretic hormone) and oxytocin. These hormones are synthesised in the cell bodies of the neurons as part of larger precursor peptides. The neurohypophysis, unlike the adenohypophysis, is not truly a gland and is actually a distal axon terminal of the hypothalamic neurons.6 Vasopressin regulates water balance and is a regulator of thirst. Water homeostasis can be maintained with as few as 10 % of the vasopressin neurons being intact. On MR T1-weighted images, the neurohypophysis or posterior pituitary appears as a bright spot and this is considered a reflection of stored vasopressin. Interestingly, the bright spot decreases with age and is usually not present in patients who have diabetes insipidus.6 Absence of the bright spot may also be seen in infiltrative diseases, such as histiocytosis, sarcoidosis and tuberculosis. Oxytocin is also secreted in the neurohypophysis and it plays a major role in childbirth and lactation.
Clinical Features of Growth Hormone Deficiency
GH is an anabolic protein that is released in a pulsatile fashion. Its main roles are to stimulate linear growth, and increase bone density and muscle strength. GH increases amino acid uptake and incorporation into protein and represses proteolysis. It has lipolytic function and activates hormone sensitive lipase, which leads to more fat being used for energy production. GH stimulates gluconeogenesis and decreases insulin sensitivity. GH also indirectly affects bone and visceral growth through somatomedins (i.e. IGFs).
In patients who have insufficient GH production (GHD), there is typically slow growth velocity in childhood. There is conflicting evidence regarding whether the birth length is normal in a child with GHD. A study of 46 infants with congenital GHD showed that length standard deviation (SD) score was normal at birth but showed deceleration in infancy.7 A child with persistent growth velocity less than the 10th percentile for age should be further evaluated. Jaundice, hypoxia, hypoglycaemia and breech deliveries have been found to be more common in patients with ectopic posterior pituitary and thin or interrupted pituitary stalk.8 Males who also have gonadoropin deficiency may have microphallus; however, the penis may be small even without gonadotropin deficiency.9
The patient’s bone age is typically delayed compared with the chronological age in congenital GHD. The patient’s skeletal proportions will correlate more with their bone age than their chronological age. Patients tend to be at lower percentiles for age with respect to linear growth compared to weight. They may appear ‘chubby’ or have a ‘cherubic’ facial appearance with flat nasal bridge and midface hypoplasia. The patient’s voice may sound younger than expected for chronological age; in addition, sparse/thin hair, delayed closure of anterior fontanelle, delayed dentition and delayed puberty may be seen. Midline defects, such as a single central incisor, lip or palate defects, absence of the corpus callosum or heart defects may also be seen. Due to the effect of GH on muscle strength, gross motor milestones may be delayed. Without GH replacement, these patients tend to have severe short stature (–4.7 to –3.1 SD below mean).10
Diagnosing Growth Hormone Deficiency
As GH is released in a pulsatile fashion, drawing random GH levels is generally not helpful. Clinical features and auxological data are essential in identifying patients who require further investigation. Biochemical testing generally involves provocation testing in response to a stimulating agent. Assays for IGF-1 and insulin-like growth factor binding protein (IGFBP-3) also play important clinical roles. Stimulation tests are the most widely used method of diagnosing GHD. Common agents used in the US and Canada include clonidine, arginine and/or glucagon. The cut-off values for diagnosing GHD varies between centres, and is dependent on the assay used and the stimuli used to induce a peak.11 Generally, at least two agents are used in provocative testing.12
IGF-1 levels <2 SD below the mean have been found to be specific for GHD; however, up to 30 % of patients with GHD may have normal IGF-1 levels. IGF-1 levels vary with body mass index, sex, age and pubertal status. Furthermore, the levels may be low in children with chronic disease or malnutrition. IGFBP-3 is specific for GHD but is not sensitive, with normal concentrations not ruling out GHD. Low IGFBP-3 levels are highly suggestive of GHD.11
Detailed neuroimaging, specifically MRI, is the most useful imaging modality for demonstrating pituitary anatomy and pathology. In addition to this, it is important to look for other abnormalities, especially of the midline, such as septo-optic dysplasia, which may be associated with congenital pituitary hormone abnormalities. MRI will also help identify tumours or other infiltrative processes in this region that can also lead to pituitary hormone abnormalities. MRI should not be used to diagnose GHD, as patients with isolated congenital GHD often have normal pituitary anatomy. Tillman et al. conducted stimulation tests and MRIs in 110 patients with GHD and found that the presence of either an ectopic posterior pituitary or hypoplastic anterior pituitary with absent stalk is highly specific (100 % and 89 %, respectively) and predictive of GHD (positive predictive value 100 % and 79 %, respectively).13 Detailed midline brain imaging is also important in determining whether a patient is at risk for multiple pituitary hormone deficiencies (MPHD), as patients with MPHD are more likely to have an abnormal MRI.3 In a patient with isolated GHD, the presence of abnormal pituitary anatomy may help guide follow-up and screening for other pituitary hormone deficiencies.
Technical Aspects of MRI in Congenital Growth Hormone Deficiency
There are several considerations to be taken into account prior to sending a child for MRI. First, it is a costly procedure and in children, depending on age, it will likely accrue additional costs for sedation and/ or general anaesthetic. Optimal images require that the patient remain still for between 30 and 45 minutes, which is not a realistic request in a young child, or in an older child with developmental delay. The bore of the magnet is small and spending a prolonged time in this enclosed space can induce claustrophobia. The machine also generates noise, which may be frightening to a young child. All patients, irrespective of age, should wear ear protectors during the procedure as recorded noise levels are as high as 85–132 dB inside the MRI machine.14–16 Contrast may need to be administered and therefore intravenous access may be required prior to the commencement of the MRI.
Neuroimaging in Isolated Growth Hormone Deficiency
MRI is the most important imaging modality in the investigation of congenital, structural pituitary gland disorders. Sagittal T1-weighted sequences (de novo or reconstructed) are used in all patients, in order to identify the posterior pituitary ‘bright spot’. In other words, the posterior pituitary is literally a bright dot and easily discernible from the anterior pituitary, which is similar in signal intensity to the adjacent grey matter. In order to detect an ectopic posterior pituitary, the radiologist then has to find this bright spot; if not in the sella, then either along the stalk or at the hypothalamus. Further planes (coronal or axial) can be useful if the posterior pituitary is initially unidentifiable on the sagittal plane. Of note, in infants, the whole pituitary will appear bright until two to three months of age. The T1 sequences we use are inversion recovery (IR) type, which provides better grey/white matter differentiation, compared with standard T1 spin echo sequences.17 This is particularly useful in infants less than six months of age.
A volumetric type sequence can be used, where all three planes can be reconstructed later during post-processing. This increases the chances of the radiologist identifying all the salient components of the gland – anterior pituitary, posterior pituitary and stalk. Technological advancements have resulted in these volumetric sequence scan times becoming shorter, resulting in ‘three planes for the price of one’. Although it could be argued that the spatial resolution is not quite as good as standard T1 sequences, we have found them to be of diagnostic quality in older children. In practice, we use sagittal and axial T1 IR type sequences in infants less than six months, and a volumetric T1-weighted sequence in older children.
The size and shape of the pituitary varies with age and sex. The gland is proportionally larger in the neonatal period than in childhood, and is generally larger in females compared with males.18 There is heterogeneity in gland shape, size and signal among normal subjects of identical age and gender.19
The most basic technique for evaluating the pituitary gland is to measure the maximum height of the gland.20 The product of the summation of the cross-sectional areas of the gland by slice-width has been shown to produce robust results in normal prepubertal children; however, the direct measures of volume do not correlate well with either one-dimensional estimates (height) or two-dimensional indirectly calculated volumes.21 As we stated previously, “numerous studies have shown variability in gland shape during childhood, making it difficult to effectively assess whether a gland is normal in size for age. Ranges of normal vary between investigators, and for this reason, defining normal values for the size of the adenohyphysis or neurohypophysis is very difficult.”3
Normal values have been established for each section of the pituitary stalk in adults.13 Unfortunately, normal values for measurement of the pituitary stalk do not exist in children. Assessment of the stalk generally depends on the experience of the radiologist. In general, if the calibre of the stalk is less than 1 mm at any point, it would be considered thin.22 Good-quality thin MR slices at the sella are essential and will generally suffice in clinical practice. The stalk could also be visualised by the use of contrast enhancement, as it and the gland will enhance avidly post-contrast.
A review of MRI findings in 15,043 children with non-acquired GHD from the Pfizer International Growth Database of patients from 1987 to 2011 revealed that abnormal MRIs were found in 4,032 (26.8 %), with empty sella (7.8 %) and classic triad (6.8 %) being the most frequent findings. There was an association between anatomical and functional abnormalities of the pituitary with ectopic posterior pituitary and pituitary stalk agenesis being more commonly associated with severe pituitary hormone defects.23 Similarly, in a study of 103 patients with congenital GHD, ectopic posterior pituitary and interrupted pituitary stalk were seen in 87.1 % of patients with severe GHD compared with 12.9 % in patients with mild-to-moderate GHD. This study also found that ectopic posterior pituitary and interrupted pituitary stalk were present in 48.6 % of patients with IGHD and 93.5 % patients with MPHD.8 Over a follow-up period of 4.5 years, 5.4 % of patients with IGHD and abnormal MRI progressed to MPHD, while none of those with normal MRI developed other pituitary hormone deficiencies.8
The variations in pituitary anatomy identified in our recent publication are consistent with previous studies.3 Of our patients with IGHD, 67 % had a normal MRI, which is similar to previous studies where normal pituitary anatomy was seen on MRI in 20–70 % of patients.2,24–26 The classic triad was seen in 60 % of patients with MPHD and 11 % of patients with IGHD. Close follow-up and long-term screening is important in patients with the classic triad and IGHD, as they may be more likely to develop additional pituitary hormone deficiencies. Our study also found that isolated anterior hypoplasia was seen in three patients with IGHD, and in no patients with MPHD. Isolated anterior hypoplasia is very rarely reported in patients with MPHD.3 From this, we concluded that a normal MRI or isolated anterior pituitary hypoplasia in patients with GHD indicates that the patient has an isolated deficiency. We also noted that an interrupted stalk was found in 66.6 % of the patients with MPHD and in 10.8 % of patients with IGHD as part of the classic triad.
Interestingly, in our study, the diagnosis of an ectopic posterior pituitary or pituitary stalk interruption syndrome was not always made at the first MRI reading, and pituitary micro-adenomas were overcalled (unpublished data). This again emphasised the importance of assessing the pituitary–hypothalamic axis on all paediatric MRI, and of knowing what pathology to look for, based on the clinical information given.
Genetics and Pituitary Anatomy
First-degree relatives are affected in 5–30 % of children with GHD, indicating that there is a genetic component.11 There are several gene defects that cause abnormal hypothalamic–pituitary development and associated hormonal dysfunction. Defects in the GH-1 and GHRHR genes lead to isolated GHD. Defects in POU1F1 and PROP1 lead to MPHD and may lead to small/normal anterior pituitary or small/normal/enlarged anterior pituitary, respectively.11 Defects in HESX1, LHX3, LHX4, SOX3, SOX2, GL12, and OTX2 and their resultant syndromes have all been implicated in abnormal pituitary anatomy and function.11 In families where there is more than one individual affected, consultation with a geneticist and possible cytogenetic testing should be considered.
Conclusions
Clinical features and auxological data are important in determining whether a patient should undergo further diagnostic testing for GHD. Provocative testing is considered the ‘gold standard’ for diagnosis, and the agents used and cut-off values vary by institution. Patients with severe GHD are more likely to have abnormal pituitary anatomy. There is a definite role for MR neuroimaging in patients with severe GHD and/or MPHD, in order to identify abnormal neuroanatomy. However, neuroimaging can be challenging in young children, as it can be a frightening experience and may require sedation or general anaesthetic. MRI of the pituitary gland in patients with congenital GHD is important in reinforcing the diagnosis and guiding endocrine surveillance. The classic triad is a more common finding in patients with MPHD than in those with isolated GHD. Patients with MPHD rarely have a normal MRI. Patients in whom there is a strong family history of IGHD or MPHD should be considered for cytogenetic testing.