Extrathyroidal production of the active form of thyroid hormone, T3, via deiodination of thyroxine (T4) provides physiologic justification for the treatment of hypothyroidism with levothyroxine (l-T4) “monotherapy”;1 T3 production outside of the thyroid gland is mediated largely by the type 2 deiodinase (D2). l-T4-treated patients exhibit stable levels of serum T32 and for decades clinicians have attributed this to adequate and consistent peripheral T4-to-T3 conversion.3,4 Accordingly, the majority of l-T4-treated patients achieve both clinical and biochemical euthyroidism, which is typically defined as normalization of the serum thyroid stimulating hormone (TSH).5,6 However, it has been recognized in more recent years that despite normalization of serum TSH, some patients suffer from residual hypothyroid symptoms and, in particular, cognitive complaints.7 Given the high prevalence of hypothyroidism, which afflicts more than eight million Americans alone,8 that about 12 % of treated individuals remain symptomatic represents a considerable portion of the population.7 Therefore, optimization of therapeutic response in hypothyroidism represents an important target for the public health.
Although l-T4-treated patients typically exhibit serum TSH, T4, and T3 levels within the normal range, this occurs at the cost of an elevated serum T4:T3 ratio.9 With that being said, a minority of patients fail to achieve normal serum T3 levels.10,11 One hypothesis to explain residual symptomatology in l-T4-treated patients is localized hypothyroidism within a particular tissue, for example, the brain. D2 is abundantly expressed within this T3-target tissue, and therefore it is logical to consider that a defect in the D2 pathway could result in localized brain hypothyroidism. If this were the case, the ability of “combination” therapy (treatment with T4+T3- containing regimens) to improve psychological parameters in some patients would be explained.12,13 However, superiority of combination therapy has not been demonstrated universally in clinical trials,5 leaving some to hypothesize that unique individual factors may characterize the subset of patients who do derive benefit from this therapeutic approach.
To this effect, a genetic factor has been associated with preference for combination therapy in hypothyroidism; patients with the Thr92Ala polymorphism in DIO2, the gene encoding D2, have demonstrated improved well-being when treated with combination therapy versus l-T4 monotherapy in a large clinical trial.14 This single nucleotide polymorphism, rs225014, results in a single amino acid substitution within D2’s instability loop.15 Given that this substitution occurs at a residue that is distant from the catalytic active site, it is not necessarily surprising that Thr92AlaD2 converts T4-to-T3 with normal kinetics when transiently expressed in cells.16,17 Similarly, these patients do not exhibit gene expression patterns consistent with hypothyroidism at the level of brain tissue,18 exhibit normal serum thyroid function parameters,17,19 and require equivalent doses of levothyroxine to normalize serum TSH levels.20,21
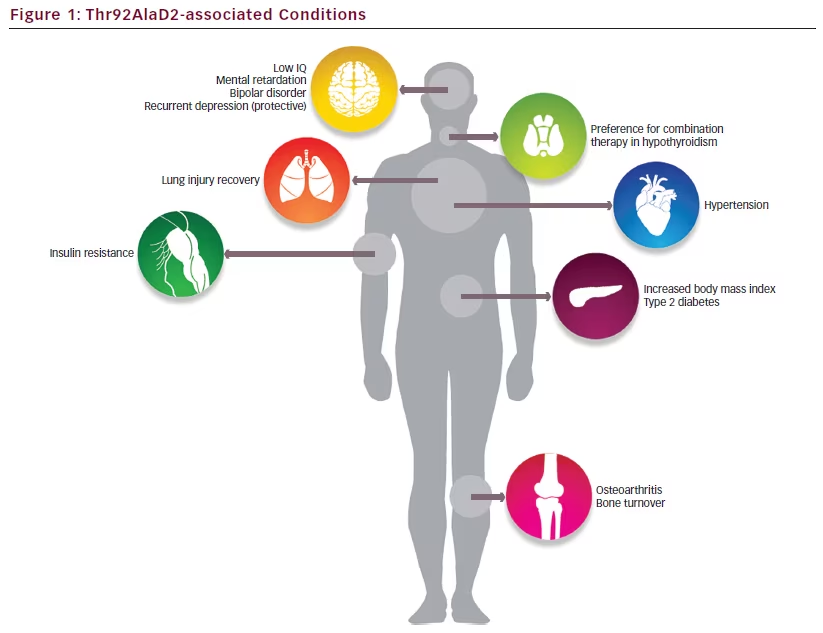
In addition to its association with treatment preference in hypothyroidism, the Thr92AlaD2 polymorphism has been associated with diverse conditions in population-based studies, including hypertension,22 insulin resistance,23,24 type 2 diabetes,25,26 bipolar disorder,27 mental retardation,28 low IQ,29 recovery from lung injury,30 osteoarthritis,31–33 and increased bone turnover34 (see Figure 1). As intact thyroid hormone signaling has been demonstrated across models, this suggests that mechanisms aside from localized hypothyroidism are likely responsible for the clinical phenotype associated with Thr92AlaD2-expression.
Translational studies have helped to define the molecular mechanism underlying the clinical phenotype associated with Thr92AlaD2- expression—stable expression of the Thr92AlaD2 protein in a human cell model modified the cellular environment such that the polymorphic protein had a prolonged half-life and was aberrantly located in the Golgi apparatus, where it perturbed Golgi morphology.18 When samples of human temporal pole were studied, there was an overlap in the gene expression patterns compared with the cellular model, suggesting for the first time that these cellular alterations might be the cause of a tissue-specific dysfunction within a D2-expressing tissue. Specifically, the transcriptome of the human temporal pole exhibited gene expression pathways that are enriched in neurodegenerative diseases.18 The concept that Thr92AlaD2-expression confers a genetic profile in the human cerebral cortex congruent with diseases such as Huntington’s suggests that further characterization of the clinical phenotype associated with
Thr92AlaD2-expression has the potential to transcend the thyroid field. Other D2-expressing tissues will also need to be studied to assess for tissue-independent patterns of transcriptional abnormalities. If carriers of the Thr92AlaD2 protein exhibit cellular perturbation throughout their D2- expressing tissues, this could explain the association with diverse clinical conditions seen in genome-wide association studies.
The concept of tissue-specific dysfunction in Thr92AlaD2 carriers should be explored in clinical trials and, in parallel, preference for combination therapy in hypothyroid Thr92AlaD2 carriers will need to be rigorously confirmed. If confirmed, translational studies will be needed to underpin the mechanism underscoring this predilection. Of course, this represents a significant frontier for future studies, but the potential for a role of personalized medicine in the treatment of hypothyroidism to improve the lives of millions of patients is of scientific and clinical priority
References
1. Werner SC, Treatment. In: Werner SC, Ingbar SH, eds., The Thyroid a Fundamental and Clinical Text, 4th ed., Hagerstown, MD: Harper & Row, 1978:965–70.
2. Surks MI, Schadlow AR, Oppenheimer JH, A new radioimmunoassay for plasma l-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement, J Clin Invest, 1972;51:3104–13.
3. Surks MI, Schadlow AR, Stock JM, Oppenheimer JH, Determination of iodothyronine absorption and conversion of l-thyroxine (T4) to l-triiodothyronine (T3) using turnover rate techniques, J Clin Invest, 1973;52:805–11.
4. Inada M, Kasagi K, Kurata S, et al., Estimation of thyroxine and triiodothyronine distribution and of the conversion rate of thyroxine to triiodothyronine in man, J Clin Invest, 1975;55:1337–48.
5. Jonklaas J, Bianco AC, Bauer AJ, et al., Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement, Thyroid, 2014;24:1670–751.
6. Wiersinga WM, Duntas L, Fadeyev V, et al., 2012 ETA guidelines: the use of l-T4 + l-T3 in the treatment of hypothyroidism, Eur Thyroid J, 2012;1:55–71.
7. Saravanan P, Chau WF, Roberts N, et al., Psychological wellbeing in patients on “adequate” doses of l-thyroxine: results of a large, controlled community-based questionnaire study, Clin Endocrinol, 2002;57:577–85.
8. Aoki Y, Belin RM, Clickner R, et al., Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999–2002), Thyroid, 2007;17:1211–23.
9. Stock JM, Surks MI, Oppenheimer JH, Replacement dosage of l-thyroxine in hypothyroidism. A re-evaluation, N Engl J Med, 1974;290:529–33.
10. Gullo D, Latina A, Frasca F, et al., Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients, PloS One, 2011;6:e22552.
11. Ito M, Miyauchi A, Morita S, et al., TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy, Eur J Endocrinol, 2012;167:373–8.
12. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ, Jr, Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism, N Engl J Med, 1999;340:424–9.
13. Hoang TD, Olsen CH, Mai VQ, et al., Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study, J Clin Endocrinol Metab, 2013;98:1982–90.
14. Panicker V, Saravanan P, Vaidya B, et al., Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients, J Clin Endocrinol Metab, 2009;94:1623–9.
15. Callebaut I, Curcio-Morelli C, Mornon JP, et al., The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure, J Biol Chem, 2003;278:36887–96.
16. Canani LH, Capp C, Dora JM, et al., The type 2 deiodinase A/G (Thr92Ala) polymorphism is associated with decreased enzyme velocity and increased insulin resistance in patients with type 2 diabetes mellitus, J Clin Endocrinol Metab, 2005;90:3472–8.
17. Peeters RP, van Toor H, Klootwijk W, et al., Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects, J Clin Endocrinol Metab, 2003;88:2880–8.
18. McAninch EA, Jo S, Preite NZ, et al., Prevalent polymorphism in thyroid hormone-activating enzyme leaves a genetic fingerprint that underlies associated clinical syndromes, J Clin Endocrinol Metab, 2015;100:920–33.
19. de Jong FJ, Peeters RP, den Heijer T, et al., The association of polymorphisms in the type 1 and 2 deiodinase genes with circulating thyroid hormone parameters and atrophy of the medial temporal lobe, J Clin Endocrinol Metab, 2007;92:636–40.
20. Santoro AB, Vargens DD, Barros Filho Mde C, et al., Effect of UGT1A1, UGT1A3, DIO1 and DIO2 polymorphisms on l-thyroxine doses required for TSH suppression in patients with differentiated thyroid cancer, Br J Clin Pharmacol, 2014;78(5):1067–75.
21. Heemstra KA, Hoftijzer HC, van der Deure WM, et al., Thr92Ala polymorphism in the type 2 deiodinase is not associated with T4 dose in athyroid patients or patients with Hashimoto thyroiditis, Clin Endocrinol, 2009;71:279–83.
22. Gumieniak O, Perlstein TS, Williams JS, et al., Ala92 type 2 deiodinase allele increases risk for the development of hypertension, Hypertension, 2007;49:461–6.
23. Mentuccia D, Proietti-Pannunzi L, Tanner K, et al., Association between a novel variant of the human type 2 deiodinase gene Thr92Ala and insulin resistance: evidence of interaction with the Trp64Arg variant of the beta-3-adrenergic receptor, Diabetes, 2002;51:880–3.
24. Estivalet AA, Leiria LB, Dora JM, et al., D2 Thr92Ala and PPARgamma2 Pro12Ala polymorphisms interact in the modulation of insulin resistance in type 2 diabetic patients, Obesity, 2010;19:825–32.
25. Nair S, Muller YL, Ortega E, et al., Association analyses of variants in the DIO2 gene with early-onset type 2 diabetes mellitus in Pima Indians, Thyroid, 2012;22:80–7.
26. Dora JM, Machado WE, Rheinheimer J, et al., Association of the type 2 deiodinase Thr92Ala polymorphism with type 2 diabetes: case-control study and meta-analysis, Eur J Endocrinol, 2010;163:427–34.
27. He B, Li J, Wang G, et al., Association of genetic polymorphisms in the type II deiodinase gene with bipolar disorder in a subset of Chinese population, Prog Neuropsychopharmacol Biol Psychiatry, 2009;33:986–90.
28. Guo TW, Zhang FC, Yang MS, et al., Positive association of the DIO2 (deiodinase type 2) gene with mental retardation in the iodine-deficient areas of China, J Med Genet, 2004;41:585–90.
29. Taylor P, Sayers A, Pearce E, et al., Effect of low thyroid hormone bioavailability on childhood cognitive development: data from the Avon Longitudinal Study of Parents and Children birth cohort, Lancet, 2014;383:S100.
30. Ma SF, Xie L, Pino-Yanes M, et al., Type 2 deiodinase and host responses of sepsis and acute lung injury, Am J Respir Cell Mol Biol, 2011;45:1203–11.
31. Bomer N, den Hollander W, Ramos YF, et al., Underlying molecular mechanisms of DIO2 susceptibility in symptomatic osteoarthritis, Ann Rheum Dis, 2015;74:1571–9.
32. Bos SD, Bovee JV, Duijnisveld BJ, et al., Increased type II deiodinase protein in OA-affected cartilage and allelic imbalance of OA risk polymorphism rs225014 at DIO2 in human OA joint tissues, Ann Rheum Dis, 2012;71:1254–8.
33. Meulenbelt I, Min JL, Bos S, et al., Identification of DIO2 as a new susceptibility locus for symptomatic osteoarthritis, Hum Mol Genet, 2008;17:1867–75.
34. Heemstra KA, Hoftijzer H, van der Deure WM, et al., The type 2 deiodinase Thr92Ala polymorphism is associated with increased bone turnover and decreased femoral neck bone mineral density, J Bone Miner Res, 2010;25:1385–91.