Primary hyperparathyroidism (PHPT) and cancer-related hypercalcaemia are the most common causes of hypercalcaemia and PHPT is the most common cause of hypercalcaemia in outpatients. An autonomous overproduction of parathyroid hormone (PTH) leading to hypercalcaemia, which is not downregulated by the calcium-sensing receptor, is the pathophysiological basis of the disease.1,2
Primary hyperparathyroidism (PHPT) and cancer-related hypercalcaemia are the most common causes of hypercalcaemia and PHPT is the most common cause of hypercalcaemia in outpatients. An autonomous overproduction of parathyroid hormone (PTH) leading to hypercalcaemia, which is not downregulated by the calcium-sensing receptor, is the pathophysiological basis of the disease.1,2
Throughout Europe and the US, PHPT is considered the third most common endocrine disease after type 2 diabetes and thyroid diseases.1–4
In Europe, population-based epidemiological studies have indicated a prevalence of PHPT of 21/1,000 in women between 55 and 75 years of age, which is an estimated equivalent of 3/1,000 of the general population.5–11 In the region of Tayside, Scotland, a population-based study showed a prevalence of PHPT of 6.72 per 1,000 population in 2006 that was greater in females than males and increased with age.6
PHPT can be caused by parathyroid adenoma, hyperplasia and carcinoma. A great amount of mechanisms contributes to the pathogenesis of this disease such as genetic predispositions due to the germline-inactivating mutations in the MEN1 and HRPT2 tumour suppressor genes. Somatic mutations in these genes were found also in sporadic parathyroid neoplasia that is a not well-known heterogeneous clinical entity.12
If possible, parathyroidectomy is the treatment of choice for clinically overt PHPT. Curative parathyroidectomy for PHPT resolves various symptoms related to the disease. For asymptomatic PHPT guidelines were developed in order to decide in individual cases between surgical and conservative approach.1–4 Management of asymptomatic PHPT includes monitoring symptoms, serum calcium, creatinine vitamin D, PTH levels and bone mineral density and also adequate calcium, magnesium and vitamin D intake as well as hydration. Medical therapy includes bisphosphonates and calcimimetics. An important question is whether patients not showing the classic manifestations of PHPT will benefit from surgery (Table 1). This question is all the more important since, among patients not surgically treated, many are lost to follow-up after 5 – 10 years and the cost of followup exceeds that of surgery.2 Parathyroidectomy led to normalisation of biochemical indices and sustained increases in bone mineral density. Without surgery, PHPT progressed in one-third of individuals over 15 years.2 These results raise questions regarding how long patients with PHPT should be followed up without intervention. Those authors against intervention base their arguments on the lack of progression in many patients and the possibility of alternative treatments should be followed up without intervention.3,4
Aetiology
The most common lesion found in patients with PHPT is the solitary, benign parathyroid adenoma, occurring in 80 % of patients. Multiple parathyroid adenomas have been reported in 2–4 % of cases and may be familial or sporadic12 and there are no clinical features that differentiate single versus multiglandular disease. Parathyroid adenomas can be discovered in many unexpected anatomic ectopic locations. The most common are within the thyroid gland, the superior mediastinum and within the thymus. Occasionally, the adenoma may ultimately be identified in the RETroesophageal space, the pharynx, the lateral neck and even in the oesophagus.
In approximately 15 % of patients with PHPT, all four parathyroid glands are involved. The aetiology of four gland parathyroid hyperplasia is multifactorial. It may be associated with a familial hereditary syndrome, such as multiple endocrine neoplasia (MEN), types 1, 2A and 4. As in the case of parathyroid adenomas, underlying molecular mechanisms are heterogeneous but with well determined germline genetic mutations. Very rarely, in less than 0.5 % of patients with PHPT the parathyroid disease will be malignant.
Hyperparathyroidism jaw tumour syndrome (HPT-JT) is associated with PHPT and fibromas in the mandible or the maxilla and tumours can also be present in the kidneys and the uterus10 (see Table 2).
Molecular Pathogenesis
Parathyroid Adenoma
The genetics, molecular pathogenesis and molecular consequences in PHPT is shown in Table 3. Although, much more information is required about the molecular pathogenesis of adenomas with PHPT, the implication of several genes so far suggests that perhaps most patients with this disorder will ultimately be shown to have some underlying molecular defect that leads to the abnormal set point for calcium. No predisposing germline DNA variants in parathyroid adenomas have been demonstrated and only a few clonally altered genes that drive parathyroid tumorigenesis have been identified. Clonal PTH gene rearrangements do not appear to be common in parathyroid adenomas. This may underestimate the true prevalence of relevant PTH rearrangements; however, it is also possible that, through other mechanisms like point mutation, a gene in the Dl1S287 region may be involved more generally in parathyroid adenomatosis. The total frequency of mutations found in sporadic primary PHPT is 9–11 %.13
In PHPT adenomas, clones of abnormal parathyroid cells emerge that dominate and shift the usual steep inverse relationship between PTH release and calcium ion ‘to the right’. For a given extracellular calcium concentration, PTH is higher. Although the defect is largely altered sensitivity of a clone of parathyroid cells to calcium, increases in the mass of abnormal parathyroid tissue also contribute to excessive secRETion of PTH. But no specific mutations of the calcium-sensing receptor have been described in PHPT adenomas and other genes, such as the vitamin D receptor gene and the proto-oncogene RET, have also not been demonstrated to be abnormal in PHPT adenomas. The clonal origin of most parathyroid adenomas implies that defects in specific genes, such as those capable of controlling parathyroid cell growth, were acquired in tumour development and conferred a selective advantage upon an original cell and its progeny. The pathogenic abnormalities in PHPT adenomas involve several genes that have variably been implicated as causal in the disorder. The first gene to be associated is the cyclin D1 oncogene (formerly PRAD1). Its overexpression, on human chromosome 11q13, is thought to have an important role in the pathogenesis of some sporadic parathyroid adenomas. The rearrangement of the PTH gene locus in proximity to cyclin D1 leads to transcriptional activation and overexpression of structurally normal cyclin D1. As many as 20–40 % of parathyroid adenomas may overexpress cyclin D1, although this overexpression is likely to vary greatly.13,14
A study confirms the frequent role of the loss of heterozygosity of chromosome 11 and MEN1 gene alterations in sporadic parathyroid adenomas and implicates a previously unassociated methyltransferase gene, EZH2, in endocrine tumorigenesis. Furthermore, genetic predisposition to sporadic presentations of parathyroid adenoma is rare to find and are probably low-penetrance germline variants in CDKI genes and, perhaps, in other genes such as CASR and AIP.15
Also, CDKN1B, encoding cyclin-dependent kinase inhibitor p27(kip1), has recently been implicated in a multiple endocrine tumour phenotype in rats and, rarely, in a human familial MEN1-like disorder.16,17 In typical, sporadic parathyroid adenomas, CDKN1B mutation can be somatic and clonal, indicative of a directly conferred selective advantage in parathyroid tumorigenesis. Additionally, the identification of germline CDKN1B variants in patients with sporadic presentations provides evidence for a susceptibility gene in the development of typical parathyroid adenomas.16–18
The results of some studies of gene HIC1 strongly support a growthregulatory role in the parathyroid glands and suggest that its perturbed expression may represent an early event during tumour development. Repressive histone modification H3K27me2/3 is involved in repression of HIC1 expression in hyperparathyroid tumours. HIC1 may act as a tumour suppressor in the parathyroid glands and whether deregulated expression involves epigenetic mechanisms.18
Parathyroid Carcinoma
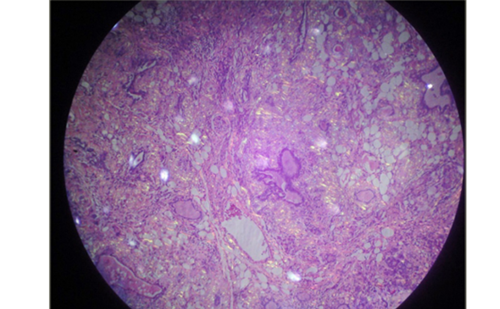
Parathyroid carcinomas are an uncommon and often devastating cause of PHPT. They characteristically result in more profound clinical manifestations of PHPT than do parathyroid adenomas. Parathyroid carcinoma is often misdiagnosed as parathyroid adenoma and long-term survival is conditioned by the extent of the primary surgical resection. Differentiating parathyroid carcinoma from degenerative changes at histopathology can be difficult and studies investigating the value of single immunohistochemical markers have had variable results. In a study aimed to investigate whether a panel of immunohistochemistry markers could aid the diagnosis of parathyroid cancer, a panel of immunohistochemistry (PGP9.5, galectin-3, parafibromin and Ki67) showed to be better than any single marker and can be used to supplement classic histopathology in diagnosing parathyroid cancer.19
Some authors consider that sporadic parathyroid carcinomas frequently have mutations that are likely to be of pathogenic importance. Certain patients with apparently sporadic parathyroid carcinoma carry germline mutations in HRPT2 and may have the HPT-JT or a phenotypic variant.13,20 Also, EZH2 contributes to the total level of aberrantly accumulated transcriptionally active (nonphosphorylated) b-catenin in the parathyroid tumour cells. EZH2 gene amplification may present the first genetic aberration common to parathyroid adenomas, secondary hyperplastic parathyroid glands and parathyroid carcinomas. If confirmed, this finding will support the possibility of a common pathway in parathyroid tumour development.21
Hereditary Hyperparathyroid States
Multiple Endocrine Neoplasia 1
MEN, both types 1,2A and 4, is inherited in an autosomal dominant manner and PHPT is often the first and is the most common of the endocrinopathies in MEN1, with high penetrance, to 95 %, during the fifth decade of life with variable clinical expressivity. On the other hand, among subjects with PHPT, MEN1 is rare, accounting for only 2 to 4 % of cases. Recognition of PHPT in an adult, nevertheless, can lead to the discovery of a kindred with MEN1. The MEN1 gene, which was identified in 1997, consists of 10 exons (localised to 11q13 ) that encodes a 610-amino acid protein referred to as menin, 67-kDa, usually expressed in the heart, but is also detected in the cytoplasm and around the telomeres. Menin is predominantly a nuclear protein that has roles in transcriptional regulation, genome stability, cell division, proliferation, DNA repair and control of cellular cycle. Germline mutations usually result in MEN1 or occasionally in an allelic variant referred to as familial isolated PHPT. MEN1 tumours frequently have loss of heterozygosity of the MEN1 locus, which is consistent with a tumour suppressor role of MEN1. The other tumours, besides the parathyroid, that can develop affect the pancreas and anterior pituitary gland. MEN1 acts as a tumour suppressor gene, such that inactivation of both alleles is required for tumour development in the target tissue (biallelic dysfunction).22,23 It is expected that about 85–90 % of the patients are affected due to germline mutations in the gene. In this regard, it is noteworthy that the de novo mutation rate is 10 % for MEN1. Since the identification of the gene, over 1,400 mutations, both germline and somatic, distributed along its entire sequence have been described.22,24 The vast majority are inactivating mutations that affect splicing consensus sites, deletions or insertions that disrupt the reading frame, and specific changes that generate shutdown signals. There are also large rearrangements present in 1 to 5 % of typical MEN1 patients, and, lastly, amino acid changes, which represent 20 % of the mutations described.25 Finally, it is worth noting that up to 30 % of patients with clinical suspicion of MEN1 mutations are not present in the gene. In this sense, various intronic mutations, which can affect the splicing process, related to the development of the disease have been described.24 In a cohort of MEN1 patients from the Groupe d’étude des Tumeurs Endocrines in France, accounting for the heterogeneity across families, the overall risk of death was significantly higher when mutations affected the JunD interacting domain. Patients had a higher risk of death from cancers of the MEN1 spectrum. This genotype–phenotype correlation study confirmed the lack of direct genotype–phenotype correlations. However, patients with mutations affecting the JunD interacting domain had a higher risk of death secondary to a MEN1 tumour and should thus be considered for surgical indications, genetic counselling and follow-up.26
Multiple Endocrine Neoplasia 2
MEN2A, in which PHPT can be associated with medullary thyroid cancer, is due to a germline mutation of the RET proto-oncogene located on chromosome 10. The oncoprotein is activated with a gain of function mutation detectable in more than 95 % of MEN2A families.27,28 MEN2 is a rare disease, with an estimated 0.5 x 106 annual incidence and prevalence of 2.5 to three cases per 100,000 in the general population. Patients diagnosed with MEN2A have a greater susceptibility to develop medullary thyroid carcinoma, pheochromocytoma and PHPT. A second subtype, MEN2B, representing approximately 40 % of the MEN2 cases are patients without parathyroid involvement. The MEN2A follows an autosomal dominant mode of inheritance with almost complete penetrance and variable expressivity. The risk of developing a PHPT is around 20 to 30 % of patients.28
The RET proto-oncogene (10q11.2), which contains 21 exons and encodes a receptor tyrosine kinase, is involved in the regulation of proliferation, differentiation and cell survival. Tumours are developed from cells derived from the neural crest stem cells and urogenital where RET is expressed. The protein has a transmembrane RET domain, extracellular binding sites each ligand, and a tyrosine kinase domain intracellular.
For MEN2, a strong genotype-phenotype correlation exists that is extremely useful when considering the clinical monitoring of the patient as well as for genetic counselling to the family will be offered. Specific phenotypes worth noting a change of cysteine to arginine at residue 634. This mutation leads to an increased risk of pheochromocytoma and parathyroid hyperplasia.29
Multiple Endocrine Neoplasia 4
Approximately 30 % of patients with the MEN1 phenotype do not carry mutations in MEN1 and this suggests that at least some are carriers of germline mutations in other loci. After identification in an animal model of CDKN1B, several groups investigated the role of the human homologous gene, CDKN1B (12p13), in families and MEN1-like negative patients for mutations in MEN1, confirming that some of them were due to germline mutations in this new susceptibility gene. This new syndrome was classified in 2007 in the Online Mendelian Inheritance in Man database under the name MEN4.
It is caused by inactivating mutations of CDKN1B gene, encoding for p27kip1 cyclin-dependent kinase 2 inhibitor, implicated in cell cycle control. Eight mutations of CDKN1B have been published in MEN4 patients. CDKN1B encodes p27Kip1 protein, which acts as an inhibitor of the cell cycle by binding to cyclin E/Cdk2 complex, preventing the phosphorylation of RETinoblastoma protein and arresting the cycle phase in G1. The mutations are scattered throughout the gene sequence, and affect the subcellular localisation of the protein, its stability or the ability to bind to p27Kip1 other proteins. Loss of expression of p27Kip1 is a frequent event in cancer and confers proliferative advantage, which can lead to the formation of a tumour.30 It has been described as a germline heterozygote frameshift mutation of the CDKN1B gene in a Caucasian woman with a long clinical history of MEN1-like multiple endocrine tumours, along with the finding of the mutation in her son and is the first report of positive CDKN1B mutation analysis in a male subject and also the first description of recurrent PHPT in MEN4.31
Hyperparathyroidism Jaw Tumour Syndrome
HPT-JT is an autosomal dominant syndrome with high but incomplete penetrance. The major features are PHPT (90 %) including 15 % of all affected by HPT-JT with parathyroid cancer, jaw tumours (30 %), bilateral renal cysts (10 %) and, less commonly, solid renal tumours. Nearly 10 % of adult cases appear to be silent carriers. Without evident jaw tumours links to the CDC73 locus at 1q25-q31, and one such 1q-linked 1.3 hereditary PHPT states was found to have occult HPT-JT based on a nonsense mutation in the CDC73 gene.32 Tumours can also be present in the kidneys and the uterus; parathyroid carcinoma is more common in HPT-JT syndrome, occurring with an incidence of up to 15–20 % of patients.33
CDC73, the gene on the long arm of chromosome 1 responsible for HPT-JT, was identified. With the ability to screen for CDC73 germline mutations. Family members at risk can be identified by DNA analysis of the CDC73 gene, which is detectable in approximately 70 % of the kindreds. These mutations result in inactivation of the gene product, parafibromin,33 a ubiquitously expressed protein that interacts with RNA polymerase II complex via pAF1. The physiological targets of parafibromin and the mechanism by which its loss of function can lead to neoplastic transformation are poorly understood. It has been show that RNA interference with the expression of parafibromin or Paf1 stimulates cell proliferation and increases levels of the c-Myc protooncogene product, a DNA-binding protein and established regulator of cell growth. This effect results from both c-Myc protein stabilisation and activation of the c-Myc promoter, without alleviation of the c-Myc transcriptional pause. It is important to note that have also been described germline mutations in CDC73 in some families with familial isolated PHPT, and up to 30% of patients with apparently sporadic parathyroid carcinoma.13,20,21
In summary, according to international guidelines, genetic testing is indicated only in patients with PHPT below the age of 30 years. However, in updated guidelines from 2012 it is suggested to perform genetic testing in patients with PHPT below the age of 30 years, but at any age in patients presenting with multigland parathyroid disease. The medical literature illustrates the importance of this change in international guidelines, but also raises concern for a potential underdiagnosing some years before.34