Diabetes mellitus is a worldwide epidemic disease, which requires continuous long-term medical care. In addition, a considerable degree of engagement is required by the patient in his or her disease management. India is now considered the diabetes capital of the world.1 The total number of people expected to suffer from diabetes will double globally from 425 million in 2017 to 629 million in 2045. It is estimated that, by 2045, 134.3 million individuals will be affected by diabetes in India, suggesting that there will be a significant increase in the population burden of disease. China (119.8 million) and the US (35.6 million) will face a much smaller rise in the numbers of people affected by diabetes.2
With the introduction of insulin to treat diabetes, a significant improvement was seen in outcomes and patients were able to achieve better glycaemic control. Better glycaemic control in turn reduces the incidence of micro- and macrovascular complications such as neuropathy, retinopathy, nephropathy and cardiovascular diseases.3 Regardless of the known benefits of insulin, physicians and patients are reluctant to practice this ‘complex therapy’.4 One out of four patients requiring insulin may refuse therapy due to psychological insulin resistance (PIR).5 In an Indian survey of 198 patients with type 2 diabetes, the major factors contributing to not starting insulin were found to be pain during injection, fear of injection and/or hypoglycaemia, social stigma and lack of education.6 With the introduction of insulin pens, patient’s perceptions of insulin therapy have gradually improved and most are able to overcome the barriers stated earlier.7 The recent Forum for Injection Technique and Therapy (FITT) expert panel on insulin injection techniques has elegantly summarised ways to address many of the barriers to insulin use.8
This observational study was conducted with patients with diabetes mellitus in North India, to understand the simplicity, safety and acceptability of insulin among patients who use pen devices for insulin delivery versus patients who use a conventional insulin vial with disposable plastic syringes. In addition, the study also took into account the cost effectiveness of the available pen devices versus disposable syringes, since India is a developing country and treating diabetes successfully demands a simple but, at the same time, cost-effective therapy.
Materials and methods
Study setting and study population
This cross-sectional observational study was conducted in the diabetes and endocrine clinic at a university-affiliated tertiary referral hospital for a period of 12 months. All patients with diabetes, who were on insulin therapy and coming in for follow up were asked if they were willing to participate in this study and were divided into two groups. One group consisted of patients injecting insulin with the use of a pen device, and the other consisted of patients injecting insulin with the use of a disposable insulin syringe. Patients from each group who gave informed consent were interviewed personally by the investigator using a self-designed questionnaire (see Tables 1–3). A pilot study was done using this questionnaire, in order to validate the questionnaire. After this, the study and the methodology was approved by the institutional research and ethics committee.
Inclusion and exclusion criteria
All patients diagnosed with type 1 and type 2 diabetes mellitus aged 16 years or older from both genders were offered a chance to participate in the study. Patients had to be using insulin either by pen or a syringe device at least once a day or for at least six months at the time of inclusion. Patients who practised self-injection and those receiving help from family members to inject insulin were included in the study.
Pregnant women taking insulin for gestational diabetes, patients using insulin pumps, patients with known psychiatric illness and patients in whom daily injections were administered by health care professionals were excluded.
Questionnaire development
A structured questionnaire was designed by the investigators (Ripudaman Singh and Jubbin J Jacob) for data collection, modifying a similar invalidated questionnaire used by Ramadan et al. among patients in Lebanon.9 The study questionnaire was divided into seven segments. The first segment consisted of general questions about the patient’s demographics as well as the type of diabetes and its duration, the duration of insulin use, the number of times blood glucose was self-monitored and the presence of any other comorbidities. The second segment had questions on the type of insulin use, method of insulin administration and the total daily insulin consumption. The third segment was designed to check the glycaemic control of these patients by reviewing the latest glycated haemoglobin (HbA1c) levels in their medical records.
The fourth and fifth segments consisted of five questions each on simplicity and safety. Simplicity was assessed by asking what the total number of missed doses were in the five days preceding the interview, the ease of handling the device/insulin during calibration of the dose, the ease of changing needles and the ease of insulin storage (Table 1). Safety was assessed by asking how painful was the process of injecting the insulin, the number of bruising episodes noted in the 5 days preceding the interview, the number of self-reported episodes of hyperglycaemia in the week preceding the interview, the number of hypoglycaemic episodes during last 3 months preceding the interview and recollection of the number of insulin cartridges/vials accidentally broken in the 12 months preceding the interview (Table 2).
The sixth segment had three questions on convenience, which was assessed by asking the total number of steps and the time taken to administer insulin. Additionally, the questionnaire enquired about the ease of administering insulin on trips and events/meals outside of the subject’s home (Table 3). The seventh segment enquired about the cost of treatment by asking each patient the average cost of their therapy per month and their opinion on their current total cost of insulin therapy (insulin, disposables and cleaning equipment).
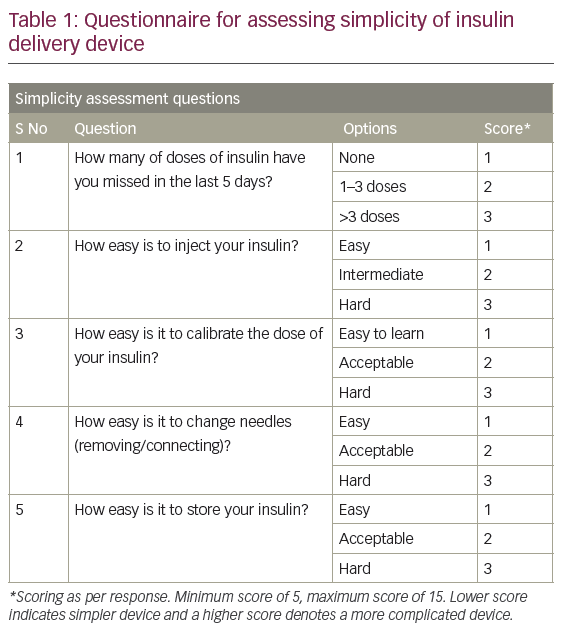

Each patient was interviewed personally by the investigator, who filled in the questionnaire based on the answers given by the patient. Each answer was given a score. The highest obtainable score for simplicity and safety was 15, whereas for convenience it was 9. The scoring system was designed to place the responses in a descending order, such that higher scores depicted a poorer response.
The questionnaire was tested by the investigator in a pilot study among 15 patients (eight syringe users and seven pen users) prior to study approval. Based on feedback, some of the questions were modified. Subsequently, the questionnaire and the study methods were approved by the institutional research and ethics committee.

Statistical analysis
A minimum sample of 60 patients (30 pen users and 30 syringe users) was determined for statistical significance using the difference in responses in the pilot study. The complete data obtained from all participants were entered in a Microsoft Excel sheet. Significant differences between sociodemographic factors and the various scores were assessed by performing a chi-square test for categorical variables and the students’ T test for continuous variables. Significance was defined as p<0.05 and all analysis was performed on Statistical Package for the Social Sciences (SPSS V.21).
Results
A total of 90 patients using insulin therapy for at least six months gave informed consent and were included in the study. Half the patients were using insulin pens for injecting insulin and the other half used disposable insulin syringes with insulin vials.
The baseline data is summarised in Table 4. More men were using insulin pen device as compared to disposable syringes (p=0.140) and the majority of patients who were using insulin syringes had used insulin syringes for over 10 years (p=0.79). However, both these differences were not statistically significant.
The average amount of insulin units administered per day was 44 ± 25.4 IU/day among insulin pen users, which was higher as compared to 37 ± 17.4 IU/day among insulin syringe users (p=0.812). Among the different types of insulin used, premixed insulin was used in a greater proportion of patients in both the groups (48.9% among insulin pen users and 62.2% among insulin syringe users).
About 22% of patients using an insulin pen device had optimal levels of HbA1c (i.e. 6–7.5%) which was higher as compared to 2.2% among insulin syringe users group. This difference in percentage was found to be statistically significant (p=0.007). The percentages of patients with optimal and poor HbA1c levels in both groups are represented in Figure 1. Mean simplicity, safety and convenience score among the pen and syringe users is shown in Table 5.
Almost all patients in both the groups felt that using the pen device for insulin administration was more expensive as compared to a disposable syringe device. The average expenditure of insulin therapy per month among pen users was Rs1,756 (Rs 1.33 per unit), which was higher as compared to Rs590 (Rs0.5 per unit) among disposable syringe users.

Discussion
Diabetes mellitus is a chronic disease which not only affects the health of an individual, but also imposes psychological impacts on their lives.10 It also greatly affects the quality of life of an individual.11
The treatment of diabetes involves the use of insulin, which is currently available primarily in an injectable form. Insulin helps to achieve better glycaemic control and significantly reduce the occurrence of diabetes-related complications.3 Despite its known benefits, the use of insulin is not easily accepted by patients due to the discomfort, social stigma and fear of hypoglycaemia associated with it.8 Every patient on insulin therapy is required to engage in frequent glucose monitoring, routine physician visits, daily monitoring of their calorie intake, and lifestyle modifications.
Since the effective treatment of diabetes utilises long-term and continuous adherence to insulin therapy, there is a need to consider patients’ preference for the type of insulin therapy. Currently, two insulin delivery devices, namely insulin pen and insulin syringe device, respectively, are commonly used.

Our study targeted several outcomes to compare insulin pen and insulin syringe devices in terms of simplicity, safety and convenience to use, also taking into account the cost effectiveness of each device. We found out that patients using the insulin pen device found it easier to learn to calibrate the dose, change the needles and store their pen device as compared to syringe users, making pen devices simple to use. Similar results were seen in a study done in Lebanon, where a higher percentage of patients from the insulin pen users group (95.2%) found the method easy to use as compared to only (46.7%) among insulin syringe users.9 The primary advantage of a simpler insulin delivery device is increased adherence and persistence with therapy. This was reflected in the safety question set by documenting missed dosing during the previous 5 days prior to the interview. This increase in adherence has been demonstrated with pen devices previously among those who switch from vials to pen devices and among older patients using pen devices.12,13
Patients using a pen device reported less pain during insulin injection, fewer incidents of bruising over the skin and minimal hypoglycaemic episodes, making the pen safer to use. These results were in concordance with a study performed in Lebanon.11 Another open-label, crossover study comparing safety profiles of insulin pen versus conventional vial/syringe for insulin injection in patients with diabetes mellitus showed similar results.14 We included hypoglycaemia episodes in the safety questionnaire as a previous study by Xie et al. has shown lower rates of hypoglycaemia when basal insulin (injection glargine) was administered with a pen device, as compared to vials.15

Patients using a pen device also reported them to be convenient to use on trips and outside the home, with less time and fewer steps involved in the injection process. The results were similar to those of Korytkowski et al., in which 85% of patients considered the use of pen to be more comfortable in public as compared to only 9% of patients using syringe device.14
Only 2.2% of insulin syringe users were maintaining an optimal HbA1C level (6.0–7.5%) as compared to 22.2% among the insulin pen users group, which was ten times higher. This is likely related to higher daily dose of insulin use among pen users and an increase in use of analogue insulins and unlikely to be related directly to pen use. However, in a study published in 2014, Xie et al. retrospectively demonstrated an improved HbA1c control among patients who were administered insulin glargine with a pen device compared to those who were prescribed the same insulin in vials.15
Almost 60% of individuals who were using a pen device for insulin delivery were males (p=0.140) and the majority of patients in the insulin syringe group had been using insulin syringes for over 10 years (p=0.79). These results could be a consequence of illiteracy and lack of awareness regarding insulin pens and poor health education in the general population, especially among women.
The average expenditure on insulin therapy among the insulin pen users group was three times higher than those using insulin syringes. This could be due to the high average amount of insulin units administered per day among pen users as compared to insulin syringe users. Moreover, among the different types of insulin used, analogue insulins were used in a greater proportion of patients from the pen group, which is costly, hence increasing the cost of therapy using a pen device. A large retrospective cohort of over 1,300 managed care patients using pen devices compared to vials suggested that the increased drug and device costs did not lead to an overall increase in total diabetes-related healthcare costs.15
Limitations of the study
The major limitation of this study is that the questionnaire was a newly developed one and has not been previously validated in other studies. The questionnaire was also only available in English and therefore had to be administered by the investigator himself, which could potentially allow for bias in the scoring. Although there was statistically significant difference in the various scores, the clinical meaningfulness of this is still debatable. We have therefore put the components of the question sets for the reader to make their own conclusion. The HbA1c values were not taken at the time of administration of the questionnaire but a recorded value closest to the interview. The difference in the type of insulin used and the dosing would also be among the many reasons for a statistical difference in HbA1c among pen users compared to syringe users.
Conclusion
In this study, more patients reported pen devices to be simpler, safer and convenient to use as compared to a syringe device. Insulin pen use was associated with better glycaemic control as compared to insulin syringe users group. Monthly expenditure on treatment among the insulin pen users group was three times higher as compared to the insulin syringe users group, which could be explained by the higher number of insulin units administered among pen users and a greater use of analogue insulins, which are more expensive. Better awareness is needed regarding the benefits of the insulin pen device over the syringe device among the population, especially women.














