Plan language summary
Background
The global COVID-19 pandemic resulted in an increased incidence of infection with Black fungus (Mucormycosis). However, the disease characteristics, chances of spread of infection and complications are not well known.
Aim of the study
We tried to find out the exact reasons behind this rise in the number of cases, whether anything particularly results in complications, and what can be done to avoid this.
Methods
We collected the medical details and treatment outcomes of patients who were managed in our hospital by various medications and surgical operations from April 2021 to August 2021. We followed up these patients for 3 months after hospital admission and discharge to identify whether any factors particularly resulted in complications, including death.
Findings
A total of 104 patients were treated for Black fungus infection during the period, of whom 88 were males. Ninety-seven patients had diabetes mellitus. Many patients who received steroid medications for the treatment of COVID-19 had worsening of their diabetes control. Even with appropriate treatment for COVID-19 and Black fungus infection, 17 patients died. Poor diabetes control, kidney problems, intensive care unit admission for aggressive treatment and surgical excision of eye(s) were found to increase the death risk. Diabetes with kidney failure was found to worsen the chance of death.
Conclusion
COVID-19 patients with more severe disease, poor diabetes control, and kidney failure have higher chance of death from Black fungus infection, especially when their eyes are badly affected by the fungus.
Mucormycosis is an aggressive mycotic infection caused by a group of multinucleated, filamentous fungi belonging to the orders Mucorales and Entomophthorales.1 In humans, infection is usually caused by the inhalation or ingestion of sporangiospores or even by traumatic inoculation of conidia via puncture wounds or trauma.1 The disease usually occurs in immunocompromised hosts and can be associated with an aggressive course with angioinvasion and tissue destruction. The mortality associated with mucormycosis can be as high as 50% (or even more), especially when patients present with disseminated and highly invasive infections.2 The angioinvasive nature of the disease often results in thrombosis and vasculitis, causing necrosis of large areas of bones and tissues.2 Devitalizing of extensive areas of skin and soft tissues further worsens the prognosis, as drug penetration is poor in these tissues.3,4
Therefore, medical treatment coupled with extensive surgical debridement is often necessary for effectively managing these patients.2,5 Immunocompromised patients with uncontrolled diabetes mellitus, organ transplantation, chemotherapy, neutropenia, deferoxamine therapy, elevated serum iron levels, corticosteroid therapy and various haematological disorders are the most afflicted group.3,6
With the second wave of the coronavirus disease 2019 (COVID-19) pandemic, India faced a sudden rise in the incidence of mucormycosis.7,8 Most of these affected patients were those with active COVID-19 infections or those who acquired the fungal invasion during the recovery phase. This itself shows a significant correlation between these two infections.9 Due to this significant rise in cases during the COVID-19 pandemic, this condition was termed COVID-19-associated mucormycosis (CAM). Furthermore, the Government of India listed CAM under notifiable diseases, whilst most of the Indian state governments declared it an epidemic.10
Compared with other systemic fungal infections, mucormycosis is a relatively rare condition; therefore, it is difficult to acquire the sample size required for conducting randomized controlled trials to study the disease’s epidemiological and prognostic characteristics effectively.11
We performed a retrospective descriptive clinical study to examine the disease characteristics and factors affecting adverse outcomes among patients treated for CAM in our hospital, and report the outcome data from this study.
Materials and methods
This retrospective descriptive clinical study was conducted among patients hospitalized with mucormycosis between April 2021 and August 2021 at the PSG Institute of Medical Sciences and Research Center located in Coimbatore, Tamil Nadu, India. The study was performed after obtaining formal institutional review board approval (reference number: PSG/ IHEC /2022/ APPR/EXP/041). The diagnosis of mucormycosis was based on clinical, radiological and/or biopsy samples of the infected tissue collected during the study period. All patients with a diagnosis were considered for inclusion in the study. Patients with a hospital stay of less than 5 days and those who opted for neither medical nor surgical management were excluded. Figure 1 is a Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) flow chart showing the detailed outline of the study.
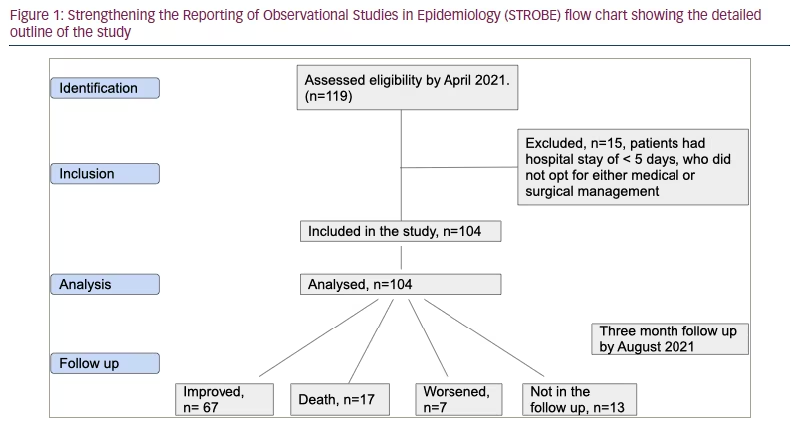
A detailed history of symptoms was obtained from every participant’s clinical chart. Clinical assessment data, including ear, nose, throat and cranial nerve examinations, and baseline haematological and biochemical investigations, were collected as part of the treatment. Every participant underwent contrast-enhanced magnetic resonance imaging of the brain and orbit, and computed tomography of paranasal sinuses to evaluate the severity of disease and tissue invasion, and these details were tabulated.
Details of the management of patients with mucormycosis are as follows. Each patient was managed with a multi-disciplinary approach, which comprised intravenous antifungal agents, surgical debridement when necessary, and treatment of underlying comorbid illnesses, such as diabetes mellitus and renal disease. A treatment protocol was formed locally in accordance with the Ministry of Health and Family Welfare of the Government of India.10 Conventional amphotericin Bb was the preferred antifungal agent at a dose of 1 mg/kg/day, owing to the very high price of the liposomal formulation in the region. For patients with renal impairment, dose adjustments were made according to the creatinine clearance (as calculated using the Cockroft-Gault equation). Patients who had moderate-to-severe renal failure (estimated glomerular filtration rate [GFR] 30–44 ml/min/1.73 m2), severe renal failure (GFR 15–29 ml/min/1.73 m2) and renal failure (GFR <15ml/min/1.73 m2) were started on the liposomal formulation of amphotericin B if they were able to afford the high expense of the treatment. The administered dose of liposomal amphotericin B was 5 mg/kg/day; a dose of 10 mg/kg/day was prescribed in case of severe disease, such as rhino-orbito-cerebral mucormycosis (ROCMrocm). Depending on the patient’s response, the intravenous antifungal treatment was continued for between 3 and 4 weeks.Details of the management of patients with mucormycosis are as follows. Each patient was managed with a multi-disciplinary approach, which comprised intravenous antifungal agents, surgical debridement when necessary and treatment of underlying comorbid illnesses such as diabetes mellitus and renal disease. A treatment protocol was formed locally in accordance with the Department of Health & Family Welfare of the Government of India.10 Conventional amphotericin B was the preferred antifungal agent at a dose of 1 mg/kg/day owing to the very high price of liposomal formulation in the region. For patients with renal impairment dose, adjustments were made according to the creatinine clearance (as calculated using the Cockroft-Gault equation). Patients who had moderate–severe renal failure (estimated glomerular filtration rate 30–44 ml/min/1.73 m2) , Severe renal failure (glomerular filtration rate 15-29 ml/min/1.73m2 were started on the liposomal formulation of amphotericin if they were able to afford the high expense of the treatment. The administered dose of liposomal amphotericin B was 5 mg/kg/day; a dose of 10 mg/kg/day was prescribe in case of severe disease, such as rhino-orbito-cerebral mucormycosis (ROCM). Depending on the patient’s response, the intravenous antifungal treatment was continued between three and four weeks.
Patients were managed medically and surgically on a case-by-case basis. Additional surgical management, which included endoscopic sinus debridement, maxillectomy, orbital decompression, orbital evisceration or exenteration, and retro-orbital amphotericin injection, was implemented depending on the extent of involvement proved by radiological imaging and clinical response to disease control. All participants were monitored clinically and biochemically every 24−48 hours for treatment response and potential complications.
After 3−6 weeks, patients were switched over to oral antifungals – posaconazole or isavuconazole (according to patients’ financial affordability of the drug, isavuconazole being costlier) for 3 months. Patients who survived the acute illness were followed up over 3 months as outpatients following hospital discharge, with repeat imaging to assess the resolution of the disease.
The clinical and laboratory data were meticulously recorded for each case using a structured proforma. The data included demographic information, clinical symptoms and signs at first presentation, imaging details, surgical treatment administered, type of antifungal and its duration, follow-up imaging details, and morbidity and mortality data.
Statistical analysis
To analyze the relationship between continuous variables, we made the initial approach of fitting logistic regression models with one covariate at a time and selecting the variables with the best fit for inclusion in the final regression analysis, along with other relevant covariates. We used advanced logistic regression to estimate the relationships between survival of patients with mucormycosis (the dependent variable) and other independent variables (i.e. age, sex, haemoglobin A1C [HbA1c] levels, chronic kidney disease [CKD], intensive care unit (ICU) admission, creatinine level at admission, presence of coronary artery disease and hypertension ). Any variable having a significant univariate test at some arbitrary level is selected as a candidate for the multivariate analysis. This statistical significance was based on the Wald test from logistic regression; a p-value cut-off point of 0.25 was selected, as more traditional cut-off points, such as p<0.05, can fail in identifying variables that are known to be necessary for our multivariate analysis (e.g. various comorbities, HbA1C levels, steroid use). Therefore, we selected all the variables whose p-value was <0.25, along with the variables of known clinical importance for the multinominal logistic regression analysis (e.g. uncontrolled diabetes, steroid use, ICU admission, presence of comorbidities).12
We assessed the probable association between the surgical procedure that patients underwent as part of the treatment of invasive mucormycosis during their hospital stay to examine if any particular treatment modality had an impact on overall survival. We also performed Cox regression analysis or proportional hazards regression, a statistical method used to model the relationship between survival time (or time to an event of interest) and one or more predictor variables. It is commonly used in medical and biological research to analyze the effects of various risk factors on time-to-event outcomes, such as the time until a patient dies or develops a particular health condition. The output of a Cox regression analysis typically includes estimates of the regression coefficients (β) for each predictor variable, as well as the hazard ratio (HR) and confidence interval (CI) associated with each coefficient. The HR indicates the relative increase or decrease in the risk of an event occurring associated with a one-unit increase in the predictor variable, while the CI provides a measure of the precision of the estimate.
Survival at 90 days was calculated from the date of diagnosis. Patients were followed up closely with enquiries over phone and frequent visits. We took an outcome analysis at the end of 90 days; if the patients were alive they underwent re-assessment, if they were lost to follow-up or had died they were categorised accordingly. Survival analysis for the continuous variable, HbA1c, was done. The survival curves were derived from Kaplan–Meier estimates. The Kaplan–Meier plots were produced in the style recommended by Morris et al. following the KMunicate study.13 They recommend to describe Kaplan–Meier curves with CIs to quantify uncertainty and also created an extended risk table (per treatment arm) depicting the number of study subjects at risk, events and censored observations over time.
The statistical analyses were conducted using the R software package and jamovi software for statistical computing. Statistical significance was set at p<0.05; all tests were two sided.
Results
During the study period, 104 cases of mucormycosis were identified, 88 of whom were men (the demographic and clinical data of the cases are detailed in Table 1). The age range was 18–80 years, and the mean (standard deviation) age was 53.69 (± 11.83) years. Of the participants, 91 tested positive for severe acute respiratory syndrome coronavirus 2 by reverse transcription-polymerase chain reaction at the time of admission, indicating active COVID-19 infection. The remainder of the cases with previous positive tests by reverse transcription-polymerase chain reaction for COVID-19 were referred from other regional hospitals. Eighty-three patients had histopathological confirmation of mucormycosis from the biopsy of infected tissue samples, and the remainder had clinical and radiological evidence for the diagnosis (staining and detection of Mucor in necrotic tissues can often be difficult and may explain negative histology in these cases).
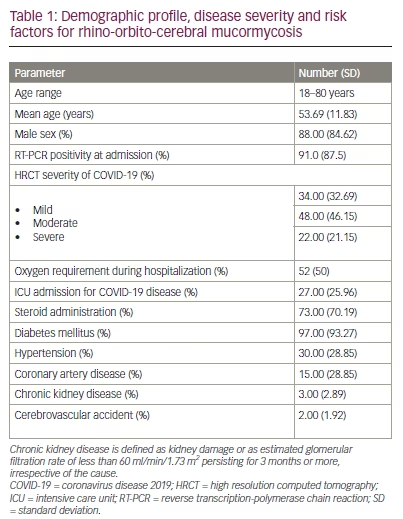
Seventy-three patients (70.19%) with moderate-to-severe disease were treated with steroids per the Indian Ministry of Health and Family Welfare guidelines for the management of COVID-19 infection.10 Seventeen patients (16.35%) died even with appropriate treatment. The baseline clinical and laboratory profiles are shown in Table 1. Of the 97 (93.27%) patients with type 2 diabetes mellitus (T2DM), 80.8% had HbA1c levels ≥6.4% at the time of mucormycosis occurrence; furthermore, 11.6% were newly diagnosed with diabetes during hospitalization. Steroid-induced hyperglycaemia (SIH) was common: 70% of patients with T2DM experienced SIH, thus requiring increased doses of antidiabetic medication or the addition of new medications for the control of hyperglycaemia.
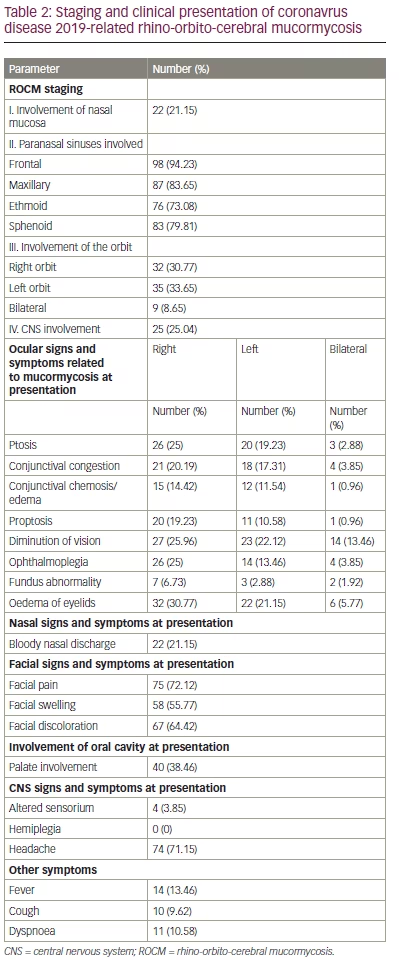
Staging of ROCM14 and its clinical features are presented in Table 2.
Histopathological diagnosis of the cases indicated invasive mucormycosis (shown by tissue and vascular invasion) in 75 cases (72.11%), and 6 cases (5.77%) also showed coinfection with aspergillosis. Surgical treatment was administered according to the severity of tissue invasion. Details of disease characteristics, treatment provided, and treatment outcomes are shown in Table 3.
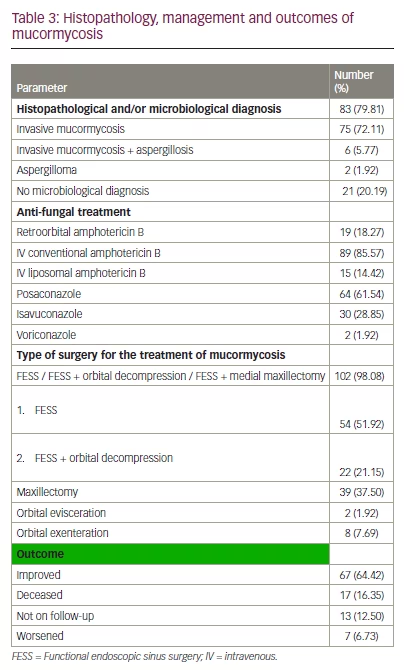
Patients who survived the acute illness were followed up for 3 months after hospital discharge and were reassessed clinically and radiologically.
The logistic regression analysis revealed that a high HbA1c level at admission had a statistically significant negative association with overall survival (odds ratio [OR] 0.79; p=0.047). ICU admission had a statistically significant negative association with overall survival (OR 0.09; p<0.001). Similarly, high serum creatinine levels at admission was also negatively associated with survival (OR 0.47; p=0.039). None of the other risk factors had a statistically significant association with overall survival at 3 months (Table 4).
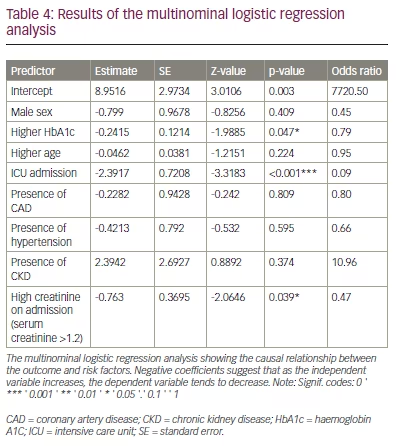
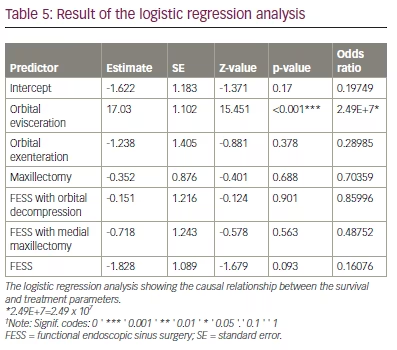
Furthermore, we assessed the probable association between the surgical procedure that patients underwent as part of the treatment of invasive mucormycosis during their hospital stay to examine if any particular treatment modality had an impact on overall survival (Table 5). None of the surgical procedures, other than orbital evisceration (OR 2.49E+7; p<0.001), appeared to be negatively correlated with survival.
Cox regression analysis revealed that higher HbA1c levels were associated with a reduced probability of survival. The overall mortality increased by a factor of 12% with each 1 percentage point increase in the HbA1c above 6.4% (HR 1.12; 95% CI 0.95–1.31). Mortality risk was found to be even higher among patients with diabetes when CKD was also present (HR 1.82; 95% CI 0.24–14.00)
The Kaplan-Meier curve was constructed by first dividing the study population into subgroups with high HbA1C and low HbA1C. Then, for each subgroup, the proportion of individuals who had survived up to a given time point was plotted on the y-axis, while the corresponding time points were plotted on the x-axis. An extended risk table (per treatment arm) depicting the number of study subjects at risk, events and censored observations over time was also created. It revealed that high baseline HbA1c (HbA1c >6.4%) was associated with low estimated survival over time, while low baseline HbA1c (HbA1c ≤6.4%) showed a higher estimated survival over time (Figure 2).
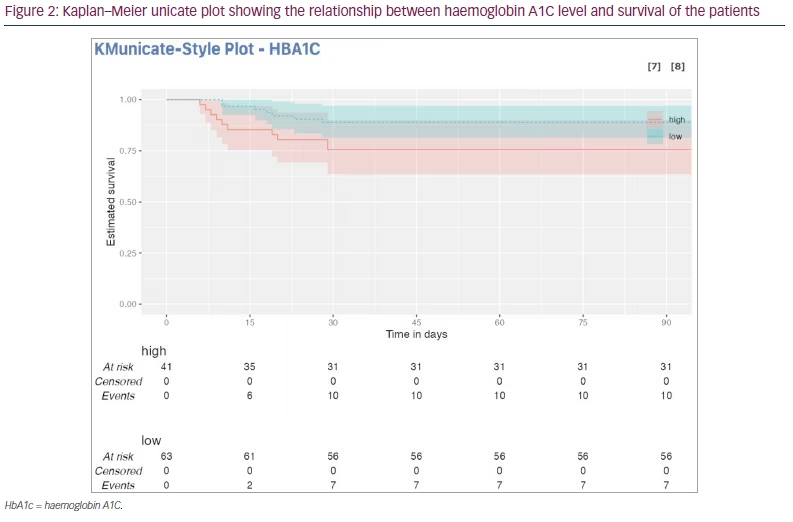
Although we analyzed the available data of our patients who were followed up for 90 days after the diagnosis of mucormycosis, no change in survival was observed after 30 days (prior to which all deaths occurred).
Discussion
This article reported the data from one of the largest single-centre studies on CAM with a robust assessment of clinical-pathological correlations, management aspects, and factors related to adverse clinical outcomes. In this study, significant predictors of mortality in patients with CAM were high HbA1c and creatinine levels, the presence of CKD, and the requirement for ICU admission for aggressive management. Orbital evisceration was also associated with significant mortality risk, probably indicating the severity of their illness.
Patients in our cohort may have been predisposed to developing CAM by preexisting diabetes mellitus or prediabetic state, steroid therapy, and compromised immune functions due to COVID-19, as observed in other studies.7,9,15 Corticosteroid use is particularly implicated in the aetiopathogenesis of CAM, as a systemic immune response to invasive fungal infections, such as mucormycosis and aspergillosis, are found in a very high proportion of patients.14 Steroid therapy may exacerbate gross alterations in lymphocyte and macrophage functions in COVID-19, and make patients particularly vulnerable to these kinds of invasive fungal diseases.
Data from our study concerning mean age at presentation (54 years) and male preponderance (88%) reflect the data from a larger study (53 years and 74%, respectively) recently conducted in India by Muthu et al. on 1,733 patients.9 The reason for male preponderance in our study is not clear; however, smoking, which is more prevalent among men,16 increases fungal invasion and thus may be a risk factor.17 Although 93.27% of our patients had diabetes or a history of diabetes, only 80.8% had an HbA1c level of ≥6.4%; this value is very similar to the prevalence of diabetes among patients with CAM reported by Muthu et al. (80.9%),9 and by Singla et al. (78.9%),18 another single-centre study from north India. However, we observed a significantly lower mortality rate (16.4%) compared with these two studies (28.6 and 33.9%, respectively).9,18 Better survival rates in our cohort. Wide variations in the morbidity and mortality risk among patients with CAM were also observed by other studies from different regions of the world.19–21
ICU admission was identified as a risk factor for lower survival rate in our study, indicating that the severity of COVID-19, as such, could have impacted the mortality risk in various studies reported worldwide. Indiscrete and prolonged use of corticosteroids among patients with COVID-19 were identified as important risk factors for CAM and CAM-related death rates.9,22,23 The widespread use of dexamethasone therapy for hypoxic COVID-19, as demonstrated by the results of the RECOVERY trial, may have led to a surge in the indiscriminate use of corticosteroids for patients with even mild disease. This could potentially explain the wide discrepancy in morbidity and mortality statistics for CAM.24
As observed in other studies,9,22,23 we also found uncontrolled hyperglycaemia in diabetes mellitus to be a significant predictor of mortality in our cohort. Similarly to other studies,25,26 our research found that patients with ROCM and diabetes had worse mortality risk when CKD or acute kidney injury coexisted. Although increasing age was a predictor of excess mortality risk in the study by Muthu et al.,9 we did not observe such an association in logistic regression analysis in our cohort, presumably because of the smaller sample size. However, we found that a better disease outcome was associated with aggressive surgical debridement of ROCM, as has been observed by others.9,27,28
We acknowledge some limitations of the present study that may affect the generalizability of our findings to day-to-day clinical practice. First, given the significant association between COVID-19 and a high risk of ROCM, it is important to note that the sample size in our study is relatively small. Second, the epidemiology of the COVID-19 pandemic has varied widely due to the presence of different strains. This may have affected its incidence, and markedly changed the clinical picture of its complications, including mucormycosis, after the second wave of the pandemic in 2021. Moreover, the risk factors and disease manifestations of COVID-19 vary significantly across different regions of the world and population subgroups;29 therefore, the presentation of ROCM and its complications may have also been different at different times and in different regions. On-going surveillance of case management from different regions of the world may provide us with more insight into these issues.
Since our study was conducted in a setting where complete data on patients was well maintained, the risk of bias in our retrospective study is not much higher than in a prospective cohort study. By themselves, Kaplan–Meier curves are not an efficient way to account for confounders in retrospective studies. Therefore, we also performed the Cox proportional hazards regression analysis appropriate for the censored survival data, to account for the measured potential confounders. Despite these limitations, our data provide broad prognostic and management perspectives to clinicians treating CAM and ROCM worldwide, highlighting the importance of our study.
Conclusions
CAM is a serious consequence of the COVID-19 pandemic and can be associated with a high risk of morbidity and mortality. Diabetes mellitus, renal impairment, and the need for ICU admission for treatment are associated with a higher risk of mortality. Early diagnosis, prompt administration of medical therapy, and appropriate surgical debridement of invasive ROCM may improve the excess mortality risks associated with CAM. Future large-scale studies are required to provide us with better insight into managing this potentially lethal COVID-19-associated disease.







