Self-monitoring of blood glucose (SMBG) levels was the standard of care for achieving tight glycaemic control in patients with diabetes for several decades. Systems for continuous glucose monitoring (CGM) were introduced around 15 years ago and are employed by an increasing number of patients. The tip of the glucose sensor used by these systems is inserted into subcutaneous adipose tissue under the skin to measure glucose in the interstitial fluid (ISF). The electrical current measured by the sensor is converted into approximate blood glucose values requiring a calibration step either by factory calibration or SMBG.1
Real-time (rt) CGM systems display the current glucose reading directly to the users, with a new glucose value displayed every 5 minutes on a receiver or the smart phone. rtCGM systems incorporate alarms that alert the user when their glucose levels are too low, too high or when a rapid rise or fall indicates a risk of glucose levels becoming too low or too high. Based on the same measurement technology, but without automatic data transfer or alarm system, the intermittently scanned (isc) CGM system requires the patient to hold the receiver close to the glucose sensor. This system does not require calibration.2,3
The adoption and real-life clinical issues associated with SMBG and CGM systems, both in daily diabetes management and in their future use with automated insulin dosing (AID) systems, were discussed at a satellite symposium, sponsored by Ascensia Diabetes Care, held at the 11th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD 2018) in Vienna, Austria in February 2018.
Glucose monitoring – insights from US and Europe
Adam Brown, Close Concerns, San Francisco, CA, US provided insights and market research data about the glucose monitoring market and technology adoption, also contrasting the situation in the US versus Europe. The market has changed dramatically in the last 5 years and is still evolving. While CGM technology continues to develop rapidly, market adoption is still moving slowly, more so for rtCGM systems than for the available iscCGM system. There is currently no agreement on which glucose-monitoring system (SMBG or CGM) should be used for clinical decisions with respect to diabetes management.
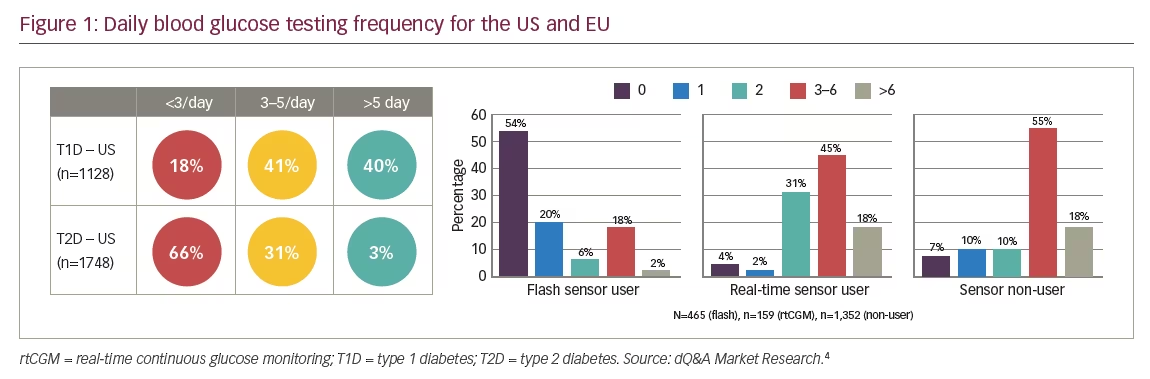
Data from the US indicates that 41% of patients with type 1 diabetes (T1D) typically check their blood glucose between 3–5 times daily and 40% >5 times daily (Figure 1).4 The majority of patients with type 2 diabetes (T2D) (66%) check their blood glucose <3 times a day.4 Across Europe, variation in daily checks was evident, and dependent on the monitoring system used.4 With the iscCGM system, 54% of patients with T1D or T2D reported performing no SMBG in a typical day and 80% reported ≤2 tests per day.4 The median number of SMBG tests was 0 per day for iscCGM users, 3 for rtCGM users, and 4 for sensor non-users.4 Nearly all iscCGM users (93%) and rtCGM users (97%) who completed this questionnaire had T1D.4 A total of 51% of glucose sensor non-users had T1D and 49% had T2D.4
The CGM market is growing, with data showing a conservative increase of around 50,000 users per quarter in the US.4 There has been a particular increase in the use of CGM in the paediatric population.4 However, the T1D Exchange Registry indicates that, in specialist centres in the US, only 24% of patients use CGM.5 It should be noted that these centres represent only a small proportion of the total diabetic population, with most seen in primary care. Globally, it is estimated that <0.5% of the diabetic population are currently using CGM.6 CGM is not yet considered standard of care, even in patients with T1D, partly due to the lack of independent, robust, randomised clinical trials demonstrating both improved outcomes for hyperglycaemia and hypoglycaemia in specific patient populations. Until recently there was a relatively insufficient evidence base supporting the accuracy and value of rtCGM in patients receiving multiple daily insulin injections (MDI) versus its use in combination with insulin pump therapy.7 However, this has now changed, with a clear benefit seen in patients with T1D using MDI.8 Additional reasons for the slow uptake of rtCGM systems has been their cost and the fact that they have to be used constantly by the patients in order to achieve a meaningful benefit.9
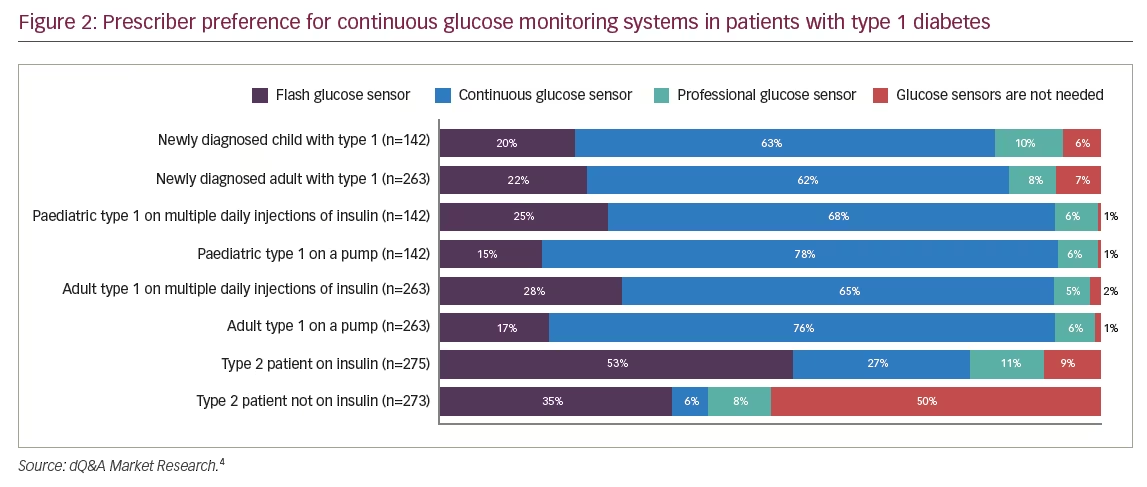
The main factor influencing choice of glucose monitoring systems for both patients with T1D and T2D in the US is insurance coverage.4 The next factor is performance. Fifteen and 9%, respectively, of patients asked, stated accuracy as a reason for choice.4 In Europe, the main reasons for choice included insurance and recommendations from doctors, diabetes educators, online reviews, and other patients with diabetes.4 Disregarding cost and coverage, a substantial minority of endocrinologists surveyed said they would prescribe CGM for their T1D patients (Figure 2).4
The insights from market research have demonstrated that most patients with diabetes perform few SMBG per day and those using CGM systems still continue to use SMBG, even with the factory calibrated iscCGM system.4 Although CGM usage is growing rapidly, it is still only reaching a minority of patients with T1D and very few patients with T2D.
Glucose monitoring in real-life practice
Guido Freckmann, Ulm, Germany, presented data about differences between measurement results obtained with SMBG systems and CGM systems, their performance standards, how limitations of CGM systems can have safety consequences in real-life, and that SMBG measurements are still necessary and important. CGM systems offer a comprehensive assessment of glycaemia, but uptake is currently limited due to costs and accuracy concerns, in addition to patient-related factors and difficulties encountered in data interpretation.10,11 Currently there is no internationally accepted standard for the measurement performance of CGM systems comparable with the International Organization for Standardization (ISO) 15197:2013 standard for SMBG systems, which specifies design verification procedures and the validation of performance by the intended users.12
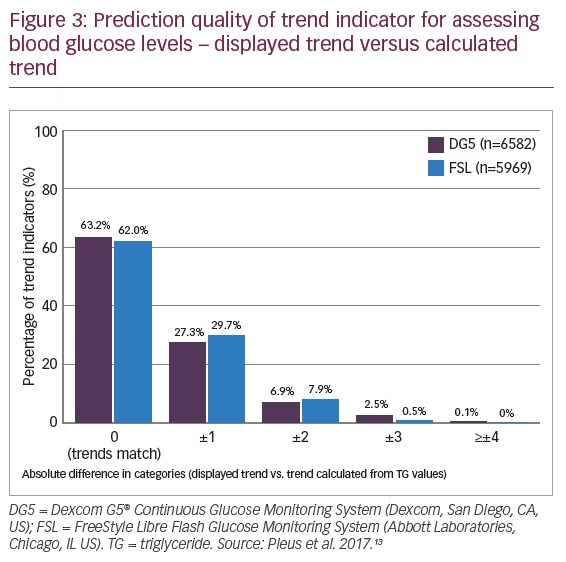
Glucose measurement by CGM systems can lack accuracy and reliability in some situations, which can limit their use in clinical practice.13 The precision of glucose measurements with CGM systems is not equivalent to that of SMBG systems, due to the higher frequency of glucose readings and additional data generated (e.g., trend information). However, the lower level of accuracy provided by CGM systems is often acceptable in daily practice. In a study evaluating the quality/accuracy of trend information, less than two-thirds of displayed trends of blood glucose levels matched calculated trends measured 15 minutes later, and around 10% of the displayed trends deviated two or more categories (Figure 3).13 With CGM, what constitutes a ‘trend’ is also not clearly defined, leading to potentially dangerous consequences.14 As a patient does not know in advance if a trend is correct, it is important for them to consider the glucose profile of the last few hours, information about meals, insulin and physical activity when making therapeutic decisions. Therefore, patients need to be adequately educated in appropriate data interpretation by a clinician with expertise in CGM or they need to participate in a well-designed training programme.
Measurements obtained with CGM systems may differ from those of SMBG systems. The reasons for such differences are not entirely clear; however, these are at least in part due to compartment measurement differences, the specific system, used and the algorithms implemented in the CGM systems.10 These differences (and associated time lags) can have an impact on measurement accuracy.1 While the effect of the time lag for newer generation CGM systems has been reduced, it can still vary from 4–14 minutes in the same patient.10 Performance of the calibration step required with rtCGM systems using high quality SMBG systems can help to ‘correct’ for physiological and sensor-related factors (e.g., sensor drift and biofouling), which also influence the time lag between the measurement of glucose in the ISF and blood.1
Issues with accuracy and precision do still arise with CGM systems, and this appears to be more problematic at the extremes of glucose excursions.15 In particular, hypoglycaemia remains a major limiting factor in achieving satisfactory glycaemic control, with patients using iscCGM spending up to five times as long in the hypoglycaemic range versus those using rtCGM (Figure 4).16 It should be noted that in most randomised controlled trials investigating CGM systems, the primary endpoint was glycated haemoglobin (HbA1c) and not a hypoglycaemia-related endpoint.17
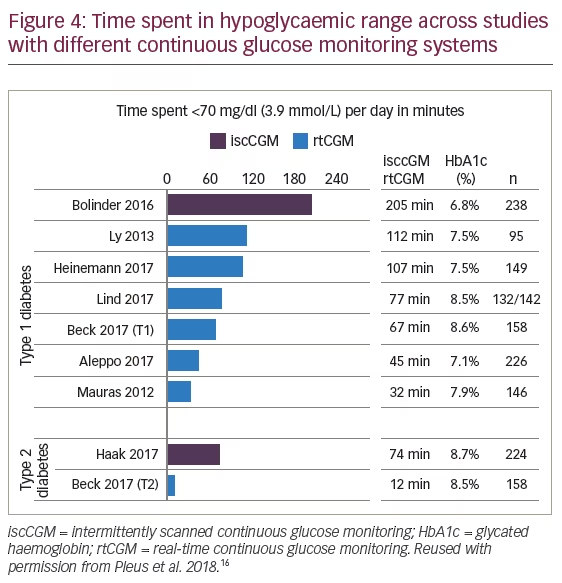
Two 6-month prospective, multicentre, randomised controlled trials, involving patients with T1D in the IMPACT study (N=241) and patients with T2D in the REPLACE study (N=224), reported a reduced time in low glucose levels when using iscCGM versus using SMBG.18 However, other studies have indicated that the measurement accuracy of iscCGM systems in the low glycaemic range is not optimal.19 Another key uncertainty around the evidence of a real benefit for patients with T1D is the fact that the IMPACT trial included only adults whose diabetes was well controlled.20
The iscCGM system currently on the market is positioned as a potential replacement for routine SMBG in diabetes management; however, both US and the EU labelling, requires that SMBG should be carried out with this device in numerous daily life situations prior to treatment decisions. This might also reflect the limited evidence for use of the iscCGM system.21, 22
The American Association of Clinical Endocrinologists and American College of Endocrinology have emphasised the need to adequately train patients who wish to use CGM systems.23 In Germany two such education programmes exist, one for rtCGM systems (SPECTRUM, which is company-independent) and one for the iscCGM system (FLASH, which is sponsored by Abbott). The American Association of Diabetes Educators state that training is essential to understand appropriate calibration of the system, as well as the factors influencing sensor accuracy, lag time of CGM values, overall interpretation of glucose trend information, and most importantly when to verify the information provided by the given CGM system to avoid potentially dangerous therapeutic decisions based on inaccurate information.24 In real-life practice, well-informed and appropriately trained patients with T1D tend to use rtCGM whereas iscCGM is more often used by patients with T2D.2,25 As patients using iscCGM perform SMBG testing only about every second day, it is important that patients are aware of the limitations of the iscCGM system and perform SMBG as recommended.
Mean absolute relative difference – looking behind the number
Marc Breton, Charlottesville, Virginia, US, discussed CGM performance characteristics, with a focus on the mean absolute relative difference (MARD). CGM data consist of a complex set of data points that are ordered in time and dependent on each other when the time interval of sampling is relatively short.26 The average or root mean square, absolute relative difference (ARD), MARD or the ISO boundaries (%20/20: the proportion of the CGM system values that are within ±20% of relative difference of reference value at glucose levels >100 mg/dL and ±20 mg/dL of absolute difference at glucose level ≤100 mg/dL) have all been used to assess CGM accuracy.12 MARD is the most commonly used accuracy index used for CGMs.27 This index is the average of the absolute error between all CGM values and matched blood glucose values measured with a reference ‘method’.2 It can be presented using the SMBG standard of at least 95% of results within 15 mg/dL of reference <100 mg/dL, or within 15% of reference ≥100 mg/dL, to evaluate the overall accuracy performance of CGM systems.12 A lower MARD percentage indicates that the CGM readings are close to the reference glucose measurement results, whereas a larger percentage indicates greater discrepancies between the two glucose measurement methods.2 There is nearly a 100% chance of satisfying ISO 15197:2013 accuracy requirements if the MARD value is between 3.3–5.3%.28 Currently, there are no CGM systems on the market that reach this level of analytical performance. Additionally, there is a high degree of variability in MARD values for different CGM systems and even with the same CGM system across different studies, with different study designs, conditions and reference methods.29 Therefore, usage of the MARD alone is insufficient to characterise the analytical performance of a given CGM system.
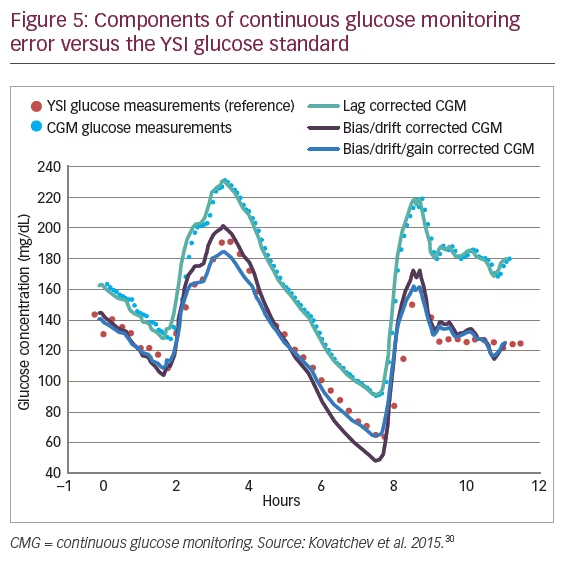
The algorithms implemented in CGM systems for improving measurement results are intended to correct a number of components of CGM measurement errors, which impact performance, including time-dependent bias, drift, delay, low-frequency fluctuations and high-frequency noise (Figure 5).30
An international consensus established by a group of experts organised by leaders of the ATTD meeting recommended that only CGM systems that provide an acceptable level of sensor accuracy should be used.8 However, MARD values are computed using clinical study data, which do not just reflect the accuracy of the CGM system, but are strongly influenced by study design.31 This explains why different CGM systems with comparable MARD values can have different clinical performance characteristics.31 Therefore, published MARD values must not be taken as a precise number reflecting the accuracy of a CGM system performance.15 The MARD only partially characterises CGM systems and may conceal significant differences. CGM systems with MARDs in the 9, 10 and 11% range have high variability, so using MARD as a single measure of analytical performance for CGMs is insufficient and could lead to incorrect conclusions.32
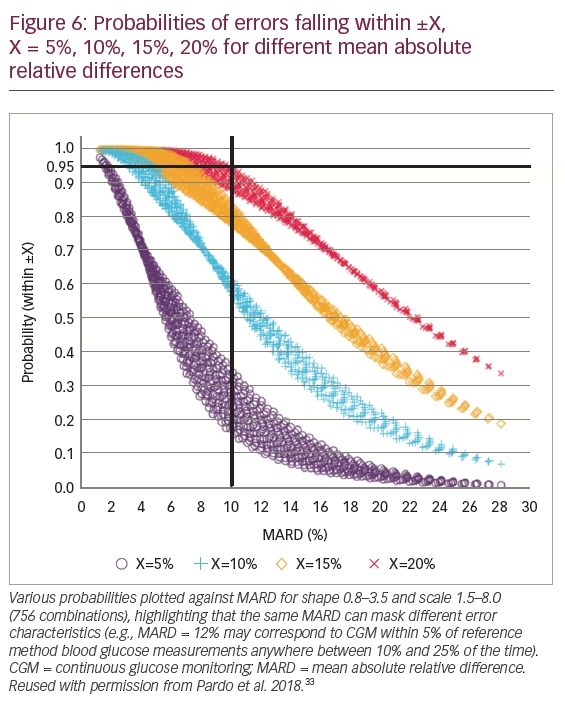
A more complete characterisation can be obtained by computing the ARD value for each CGM system and reference blood glucose measurement pair for each subject/sensor combination.32 This methodology has been used in a study to assess CGM measures of accuracy.32 Empirical distributions (normal, lognormal, Weibull, Johnson [SU, SB, and SL] and gamma) of ARD for each subject were computed, and parametric forms were fit to these distributions. The gamma distribution was chosen as the best model for ARD.32 A linear relationship between the probability that an ARD is less than or equal to a given value (e.g., 15%) and MARD was demonstrated.32 When a range of probabilities were plotted against MARD for shape 0.8–3.5 and scale 1.5–8.0 (756 combinations), the same MARD was shown to mask different error characteristics (Figure 6).32,33 At 15% (which is considered the ISO standard), CGMs with a MARD of 10% had high variability. Sensor data indicated that the sensor was within 15% of the reference between 78–91% of the time.33
Additional measures of analytical performance are needed to provide a more complete characterisation of CGM system performance. The error probability calculation facilitated by the gamma distribution model may be used in addition to MARD to provide a more informative tool for assessing CGM system analytical performance.
Importance of accurate blood glucose measurements for artificial pancreas
Steven Russell, Boston, MA, US presented information on the interdependency of SMBG and CGM systems and the role of accuracy of these systems for a reliable performance in AID systems. The current management of diabetes requires intensive efforts by patients to count carbohydrates, closely monitor blood glucose values and make insulin dosing decisions, a drug with a narrow therapeutic window and a low margin for error.34 These collectively indicate an unmet need for better methods in diabetes management.
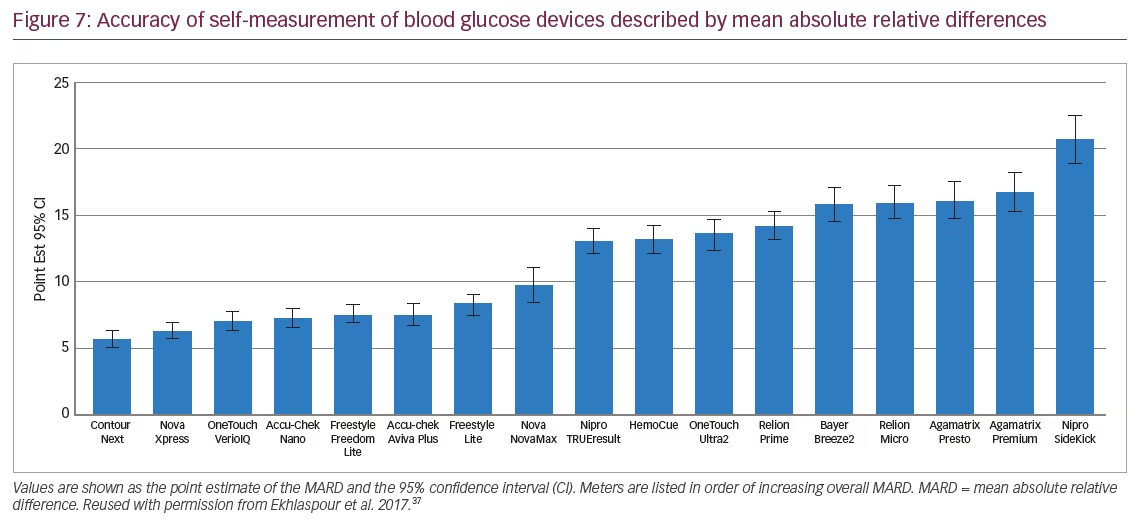
Usage of CGM systems has been shown to improve overall glycaemic control (lower HbA1c and frequency of hypoglycaemic events) when combined with MDI or usage of insulin pumps.35,36 However, the accuracy of rtCGM systems depends critically on appropriate performance of the calibration procedure once per day by means of SMBG. Measurements with the iscCGM system depend on the accuracy of the initial factory calibration, which does not address a patient’s individual characteristics that influence overall device performance. The accuracy of SMBG systems themselves is also critical. In a recent independent review of 17 commercially available, commonly used glucose meters and their respective test strips, from nine manufacturers, accuracy was found to be highly variable (Figure 7), exhibiting a range of MARDs from 5.6–20.8%.37 Only some SMBG systems were robust and exhibited a high degree of accuracy.37
One of the most exciting developments for the treatment of diabetes is the AID system, sometimes referred to as a closed-loop system, which aims to imitate the function of a healthy pancreas by automatically varying (without patient intervention) the subcutaneous insulin infusion rate very frequently, depending on the current glucose values. It comprises an rtCGM system, an insulin infusion pump and a SMBG device to calibrate the rtCGM or supplement the AID system when no rtCGM data are available.38 While the AID system holds promise, it adds challenges in terms of safety since it combines several components into one system and takes over the glucose control from the patient. The efficacy and safety of these devices is inherently dependent on the accuracy and reliability of each of the individual components.39 The US Food and Drug Administration (FDA) state that, “Because the accuracy of the BGD [Blood Glucose Device] exerts a tremendous impact on the quality of the calibration and the performance of the APDS [artificial pancreas device system], sponsors are encouraged to consider use of the most accurate BGD devices that are practical for patient use.”39
In September 2016, a (Hybrid) Closed Loop System (Medtronic MiniMed™, 670G hybrid closed-loop; Medtronic, Minneapolis, MN, US) became the first AID system to be approved by the FDA.40 This system requires a minimum of two, and on average four, calibration measurements each day, and has been associated with few serious or device-related adverse events in patients with T1D.41 The SMBG used (CONTOUR® NEXT LINK 2.4; Medtronic, Minneapolis, MN, US), was required to meet the FDA’s high Class III/pre-marketing approval standards for the system.
A number of other AID systems are being evaluated for use in patients with T1D, including Tandem, Bigfoot Biomedical, Beta Bionics and Insulet. According to experts, the calibration-free AID system is still a long way off. The ‘bionic’ pancreas will target and manage glucose levels close to the hypoglycaemic range and will require tighter tolerance and controls. A difference of 15 mg/dL in accuracy could result in a reading of 70 mg/dL being 55 mg/dL. At this tight range there is no margin for error and system accuracy matters.
Conclusions
Technological solutions for glucose monitoring in patients with diabetes have been improved during the last decades, and reliable systems for SMBG and CGM now exist. However, improvement in a number of areas is still needed, including the need for recalibration, variability in glycaemic patterns and lack of standardised software methods for analysis of CGM data, which mean CGM utilisation in daily practice is currently limited. There is also a lack of randomised controlled trials, and protocols for patients to monitor the trends in glycaemia are not available. With the existence of performance standards, standards for analysis of CGM data and high-quality training programmes for patients (and physicians), usage of CGM systems is possible. Availability of accurate SMBG systems remains a cornerstone in diabetes management, and also for usage in AID systems.







