Polycystic ovary syndrome (PCOS), the most common endocrine disorder in women of reproductive age, is a heterogeneous androgen-excess disorder with different degrees of reproductive and metabolic dysfunctions.1 Thyroid disorders are also quite common and are among the most common endocrine disorders worldwide.2,3 Subclinical hypothyroidism (SCH) is defined as an elevation of serum thyroid-stimulating hormone (TSH) levels beyond the normal range despite normal serum levels of free thyroxine (FT4).4 SCH, or mild thyroid failure, is a common problem found in 4–10% of the overall population; however, in young women aged 12–39 years, the prevalence is 2%.2,3 Menstrual disturbances, oligo/anovulation, subfertility, miscarriage and polycystic appearance of the ovaries are common features of PCOS and overt hypothyroidism; for this reason, overt hypothyroidism must be excluded for establishing the diagnosis of PCOS.1 Many of these reproductive manifestations of PCOS could also be present in SCH.5 Though PCOS and SCH have completely different aetiopathogenesis, they have multiple common metabolic presentations.6 Insulin resistance appears to be important in the pathogenesis of PCOS; subjects with overt hypothyroidism and SCH also demonstrate insulin resistance.1,6,7 Obesity, dysglycaemia, dyslipidaemia, metabolic syndrome and increased risk of cardiovascular (CV) diseases have been observed in both PCOS and SCH.8–11 High prevalence of SCH has been observed in PCOS in recent studies, and the prevalence is higher than the age-matched healthy population.12,13 As both PCOS and SCH are related to increased CV risk, the presence of SCH in patients with PCOS may amplify CV risk in such patients. Previous studies have provided inconclusive results as to whether the presence of SCH in patients with PCOS has a higher CV risk than in patients with PCOS without SCH.13–16
Data regarding the effect of SCH on clinical, hormonal and metabolic parameters in PCOS are insufficient, particularly within the context of Bangladesh. This study was conducted to explore whether the presence of SCH imparts higher reproductive and metabolic derangements in women with PCOS.
Methods
This cross-sectional study was conducted among newly diagnosed patients with PCOS attending the endocrinology outpatient department of Mymensingh Medical College Hospital, Bangladesh, from January 2017 to December 2019. The protocol of the study obtained approval from the institutional review board of the hospital. Patients were included if they had a diagnosis of PCOS meeting the revised Rotterdam criteria, 2003; the diagnosis of PCOS in adolescent girls was made based on the presence of clinical and/or biochemical evidence of hyperandrogenism (after exclusion of other pathologies) in the presence of persistent menstrual irregularities.1,17 Pregnant and lactating patients, known cases of congenital adrenal hyperplasia, those with pelvic inflammatory disease, and those with any malignancy, were excluded. Patients with a known history of thyroid dysfunction, history of thyroid surgery or radio-iodine ablation and those taking drugs that may interfere with thyroid function were also excluded. Patients on treatment for PCOS, those on hormonal contraception or those taking metformin or any drug for PCOS or hirsutism were also excluded. Hirsutism was assessed by the modified Ferriman–Gallwey (FG) score: a score of ≥8 was used as the cut-off point for diagnosis of hirsutism.18
A semi-structured, questionnaire-based interview was conducted by the investigators on a one-to-one basis with study participants, in order to gather detailed information on clinical presentation and family history. Anthropometric measurements were collected in the outpatient department by investigators. Fasting oral glucose tolerance test (OGTT) was conducted and fasting lipids were measured in all patients. For cultural reasons, transvaginal ultrasonography was conducted in married patients, whereas transabdominal pelvic ultrasonography was done in those who were unmarried. Serum TSH, FT4, anti-thyroid peroxidase (anti-TPO) antibody, total testosterone, and prolactin were measured by automated hormone analysers using the radioimmunoassay (normal reference range of the corresponding laboratory for TSH was 0.3–5.0 µIU/mL and for FT4 was 0.71–1.85 ng/dL).
SCH was diagnosed as elevated TSH and normal FT4 levels according to the American Thyroid Association guidelines.4 Obesity status was determined by body mass index (BMI) categories applicable to Asian Indians.19 Central obesity was defined as waist circumference ≥80 cm in the adults and adolescents aged >16 years; in the adolescents aged 10–16 years, waist circumference ≥90th percentile (or adult cut-off if lower) for Indians was used.20,21 Hypertension and pre-hypertension were defined according to the Joint National Committee VII criteria.22 Prediabetes and diabetes mellitus were diagnosed according to criteria described by the American Diabetes Association.23 Dyslipidaemia was defined according to cut-off points described in Adult Treatment Panel III.24 Metabolic syndrome in adults and adolescents aged >16 years was diagnosed by using the International Diabetes Federation (IDF) criteria applicable to the South Asian adult women.20 In adolescents aged 10–16 years, metabolic syndrome was diagnosed following diagnostic criteria proposed by the IDF for the diagnosis of metabolic syndrome in this age group.25
For analysis, study participants were split into two groups, those with SCH and those who were euthyroid. Statistical analysis was done using Statistical Packages for Social Sciences for Windows, version 23.0 software (SPSS Inc.; Chicago, IL, USA). The categorical variables were presented as percentages; measurable variables with normal distribution were presented as mean ± standard deviation (SD), and those not following normal distribution were presented as median. Student’s t-test, Chi-square test and Mann–Whitney U tests were performed as applicable to compare the variables between the two groups. A p value ≤0.05 was considered to be statistically significant.
Results
Four hundred and sixty-five women with PCOS, aged 12–40 years (mean age 22.52 ± 5.38 years), were evaluated in this study; almost half of them (47.7%) were married. The frequency of SCH was 10.8% (50/465), and 18.3% (85/465) of the study subjects were positive for the anti-TPO antibodies.
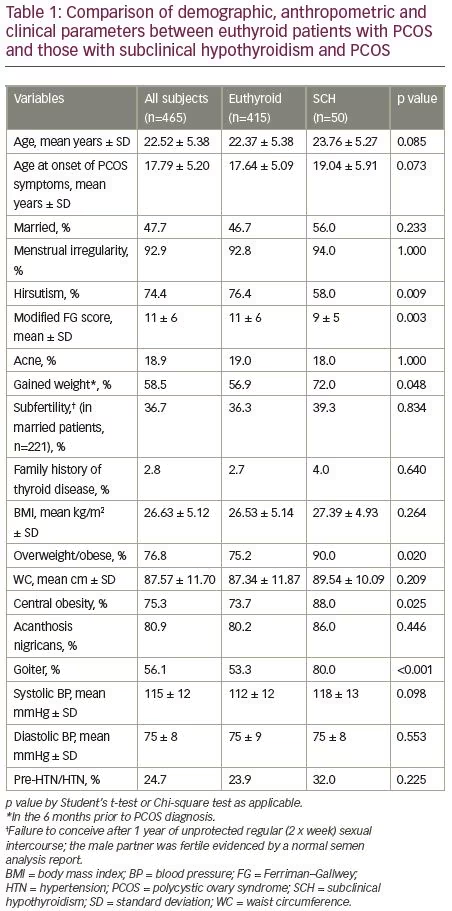
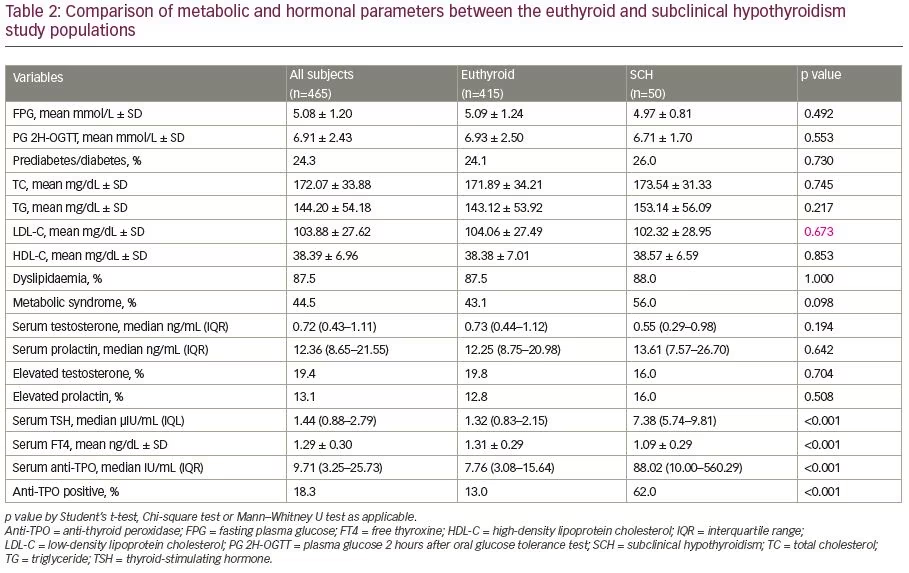
The demographic, anthropometric and clinical parameters of the study subjects are shown in Table 1. The euthyroid and SCH groups did not differ in the majority of these variables except for a lower modified FG score, hirsutism frequency and higher frequencies of overweight/obesity, central obesity and goitre in the SCH group than euthyroid group. The metabolic and hormonal parameters were also almost similar in the SCH and euthyroid groups with the exception of a higher TSH, anti-TPO, thyroid auto-immunity and lower FT4 in the SCH group compared to the euthyroid group (Table 2).
Discussion
In this study, conducted in a tertiary hospital in Bangladesh among 465 patients newly diagnosed with PCOS, it was observed that 10.8% of participants had SCH and 18.3% were positive for the anti-TPO antibody. Study participants with PCOS and SCH had a lower frequency of hirsutism and a lower modified FG score than the euthyroid patients; the frequencies of overweight/obesity, central obesity and goiter were higher in the SCH group. Autoimmune thyroid disease, as measured by anti-TPO positivity, was higher in the SCH group. Other clinical and metabolic parameters were statistically indifferent in the two groups.
Thyroid disorders and PCOS are common endocrine disorders. Although these two are clinically separate entities, they share many clinical and radiological abnormalities, including menstrual irregularities, infertility, spontaneous abortion, obesity, lipid abnormalities and enlarged ovaries with polycystic morphology.26,27 Though the exact links connecting the pathophysiology and clinical manifestations of PCOS and thyroid disorders have not been established, there are some common factors which predispose an individual to both disorders, in addition to a pathophysiological connection between the two disorders.27 Despite this uncertainty in disease connection, it is now evident that thyroid disorders are more common in women with PCOS compared with age-matched healthy women.12–14 Both genetic and environmental factors are believed to contribute to thyroid disorders in PCOS.12 Hypothyroidism is also known to cause PCOS-like ovaries and overall worsening of PCOS and insulin resistance.12
In the current study, 10.8% of participants with PCOS also had SCH, which is almost similar to the findings of Benetti-Pinto et al., who found that 11.3% of Brazilian women with PCOS have SCH.14 However, this frequency is lower than the observed prevalence of SCH in PCOS in India by Sinha et al. (22.5%), in China by Yu et al. (27.0%), and in Iran by Enzevaei et al. (25.5%).12,13,28 Data on the prevalence of SCH in the general population in Bangladesh are lacking, though it is presumed to be 10%.29 From previous studies, it is also known that SCH is less frequent in younger people than the overall population.2,3 Therefore, the frequency of SCH in women with PCOS aged 12–40 years in the current study may be higher than the prevalence of SCH in the healthy women of the similar age in Bangladesh.
The observed frequency (18.3%) of anti-TPO positivity in the current study is lower than that observed in other countries, e.g., 22.5% documented in India, 25.0% in China, and 26.9% in Germany.12,13,30 In the absence of a healthy control group, and also with the unknown prevalence of autoimmune thyroid disease in Bangladesh, we are unable to comment on whether this frequency of thyroid autoimmunity is higher than age-matched controls; though in one study conducted among patients in Bangladesh, the prevalence was found to be very high (32.9%).31
Both PCOS are SCH are associated with some common female reproductive adverse outcomes, including irregular menstruation and subfertility.1,5 Though the presence of SCH in PCOS imparts a higher risk of these reproductive problems, the frequency of menstrual irregularity was similar in patients with PCOS with or without SCH in this study. Trummer et al.’s observation was similar in this regard.32 Euthyroid patients with PCOS had more irregularities in their menstrual cycles compared with those with PCOS and SCH in the study by Enzevaei et al.28 We observed a slightly higher frequency of subfertility patients with SCH than their euthyroid counterparts, though the difference was not statistically significant; other researchers also had similar observations.14,28
Thyroid dysfunction is known to affect female reproductive hormone levels. Sex hormone-binding globulin decreases in hypothyroidism and its binding affinity is altered; the metabolic clearance rate of testosterone is also decreased, ultimately resulting in a decrease in total testosterone and an increase in unbound testosterone.33 In this study, the differences in the features of hyperandrogenism were inconstant between the two groups: subjects with PCOS and SCH had a lower FG score and hirsutism frequency than those without SCH, while both groups had similar total testosterone levels, frequencies of acne and biochemical hyperandrogenism. Euthyroid patients with PCOS and those with SCH and PCOS were found to have indifferent FG scores and hirsutism frequency by Yu et al., Benetti-Pinto et al., and Enzeveai et al.13,14,28 The frequency of hirsutism was higher in euthyroid PCOS than in SCH–PCOS in the study done by de-Medeiros et al.15 A similar frequency in the presence of acne in the two groups was observed by Enzeveai et al., but de-Medeiros et al. found a higher frequency of acne in patients with PCOS with SCH.28,15 Similar total or free testosterone levels were observed in the two groups by Yu et al. and Benetti-Pinto et al., and a meta-analysis also had similar observations.13,14,16 Free testosterone was higher in euthyroid subjects with PCOS compared with SCH–PCOS in the study by Enzeveai et al.28
Both PCOS and SCH are associated with metabolic dysfunctions. Both are associated with clinical and biochemical markers of metabolic syndrome, including acanthosis nigricans, obesity (particularly central obesity), hypertension, abnormal glucose tolerance and dyslipidaemia. It is assumed that presence of SCH in PCOS may be associated with higher metabolic risks.1,5,10 In this study, we found that the euthyroid PCOS and SCH–PCOS groups had similar frequencies of acanthosis nigricans. Higher frequency of acanthosis nigricans was observed in women with PCOS with thyroid dysfunction than in women without thyroid dysfunction in a study by Mustari et al.34 Though a higher number of subjects with SCH gain weight during the course of PCOS, BMI was similar in the euthyroid and SCH groups in our study. But, interestingly, more subjects in the SCH group were overweight/obese (BMI ≥23 kg/m2). The differences in BMI in similar patient populations were insignificant in the studies by Yu et al., Benetti-Pinto et al., de-Medeiros et al., and Enzevaei et al.13–15,28 In a meta-analysis, BMI was also found to be similar among euthyroid patients with PCOS and those with SCH and PCOS.16 On the contrary, Yasar et al. observed that the increased prevalence of SCH in women with PCOS might be the result of increased BMI.35 In line with the findings of Benetti-Pinto et al.,14 de-Medeiros et al.15 and Enzevaei et al., we also observed no significant differences in waist circumference between the two groups, though the SCH group had a higher frequency of central obesity in our study.28
SCH is associated with increased systolic and diastolic blood pressure.10,36 Hypertension is also more prevalent in women with PCOS compared with the general female population.37 It might be assumed that SCH in PCOS may impart an additional risk of hypertension; however, interestingly, no differences in blood pressure or in the frequency of hypertension have been observed in patients with PCOS with and without SCH by previous researchers.13–15 We also had similar observations.
Insulin resistance is the key pathogenic feature of PCOS and dysglycaemia (diabetes and prediabetes), and commonly complicates PCOS.1,6 Patients with overt hypothyroidism and SCH have demonstrated both insulin resistance and diminished early insulin secretory response, resulting in prediabetes or diabetes mellitus.7,9,11 The presence of these two risk factors of insulin resistance and dysglycaemia might be expected to have an additional influence on glucose metabolism, though the results of previous studies are not uniform in this regard.14–16,28
Benetti-Pinto et al., de-Medeiros et al., Enzevaei et al. and Huang et al. found similar fasting plasma glucose (FPG) in such groups; PG 2H-OGTT values were also similar in the two groups in the study by de-Medeiros et al.14,15,28,38 Glucose intolerance was also not statistically different between those with PCOS and those with PCOS and SCH according to the observation of de-Medeiros et al.15 On the contrary, FPG levels were significantly higher in patients with PCOS and SCH when compared with euthyroid patients with PCOS in a meta-analysis.16 We observed no differences in FPG and PG 2H-OGTT values between the euthyroid and SCH-PCOS groups, and the frequency of dysglycaemia (prediabetes/diabetes) was also similar between the two groups.
Both PCOS and SCH are associated with lipid abnormalities.1,8,10 In addition to elevations in triglycerides and decreases in HDL-cholesterol, women with PCOS have higher LDL-cholesterol and non-HDL-cholesterol, regardless of their BMI.39 In our study, participants in the euthyroid and SCH groups had indifferent lipid parameters (total cholesterol, triglyceride, LDL-C and HDL-C), and the frequency of dyslipidaemia was also similar in the two groups. Differences in lipid parameters in similar patient populations have not been uniform in previous studies. de-Medeiros et al. and Enzevaei et al. found comparable lipid parameters between the two patient groups.15,28 Yu et al. reported that total cholesterol, LDL-C and triglycerides were significantly higher in patients with SCH and PCOS compared with euthyroid patients with PCOS, though HDL-C levels were similar.13 Benetti-Pinto et al. observed similar total cholesterol, triglycerides and HDL-C in euthyroid patients with PCOS and those with SCH and PCOS; however, LDL-C levels were higher in the SCH group.14 In a meta-analysis, it was found that patients with SCH and PCOS have higher triglycerides, higher total cholesterol, similar LDL-C and lower HDL-C levels compared with their euthyroid counterparts.16 Yu et al. and Huang et al. observed a higher prevalence of dyslipidaemia in patients with PCOS with SCH when compared with their euthyroid counterparts.13,38 Separately, both PCOS and SCH are associated with increased risk of metabolic syndrome.1,8,10 In this study, though a slightly higher frequency of metabolic syndrome was observed in the SCH–PCOS group than the euthyroid PCOS group, the difference was not statistically significant. In this regard, it might be pertinent to mention that a similar homeostasis model assessment of insulin resistance index (HOMA-IR) in euthyroid patients with PCOS and those with SCH and PCOS was demonstrated in a recent meta-analysis.16
Hyperprolactinaemia is observed in both SCH and PCOS, and we observed similar prolactin levels in the euthyroid and SCH groups; the frequency of hyperprolactinemia was also similar in the two groups.1,5 de-Medeiros et al. and Mustari et al. reported a similar observation to our study, while Benetti-Pinto et al. found higher prolactin levels in women with PCOS who had SCH.14,15,34
This study has some limitations. It was a single-centre study; the sample may not represent the whole country. The sample size was also small; only 50 patients with SCH and PCOS were compared with 415 euthyroid patients with PCOS, which may influence the between-group variables. No healthy control group was included, limiting the comparison with patients with PCOS. Nevertheless, this is the first study in this country describing the differences of clinical, metabolic and hormonal parameters in patients with PCOS with and without SCH. The study results may serve as the basis for further large-scale, multi-centre studies in this subject.
Conclusions
The similar adverse reproductive and metabolic consequences in women with PCOS with or without SCH in this study clearly indicates that these consequences are due to PCOS itself; the additional presence of SCH in these patients may not impart additional risks. However, these findings should be interpreted with caution, as large-scale studies investigating the basic pathophysiological effects of SCH on PCOS are currently lacking.







