The past decades have seen the introduction of a variety of oral antidiabetic drug (OAD) classes and drugs. The initial straitjacket approach, using traditional sulfonylureas and biguanides, has given way to a more flexible (and confusing) choice of drugs. Sulfonylureas, pioglitazone, dipeptidyl peptidase-4 (DPP-4) inhibitors, sodium-glucose co-transporter-2 (SGLT2) inhibitors, bromocriptine and colesevalam all vie for a place under the glucose-lowering sun. While this choice is welcome, it also brings controversy and confusion.
Sulfonylureas – stars under attack
Availability of the new drug classes has led to a debate regarding the utility and viability of sulfonylureas as a therapeutic option. Uninformed opinion views the sulfonylureas as a homogenous group of drugs, with high risk of hypoglycaemia and cardiovascular complication. While this may be true for older sulfonylureas such as tolbutamide and chlorpropamide, these arguments are not valid for modern sulfonylureas.1–3
The modern sulfonylureas are proven to be effective, safe and well tolerated in a variety of clinical situations. Gliclazide modified release (MR) has been documented to improve cardiovascular outcomes, offer a beneficial glycaemic legacy, and increase lifespan. Robust evidence, gained through studies such as Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE),4 ADVANCE-ON5 and Steno-2 trials,6 enable us to dispel the challenge of misinformation that modern sulfonylureas face.
Confusion regarding choice of second OAD, however, still persists. The American Diabetes Association (ADA) lists four non-insulin alternatives for patients with type 2 diabetes and metformin inadequacy. These are sulfonylureas, pioglitazone, DPP-4 inhibitors and SGLT2 inhibitors. However, it does not offer guidance regarding how to choose a particular class.7 The ADA and American Association of Clinical Endocrinologists8 group all sulfonylureas into one class, without taking their dissimilarities into account. This simplistic choice of framework prevents the advantages of modern sulfonylureas from being highlighted.
The concept of flexibility
The confusion surrounding sulfonylureas can be dispelled by discussing the concept of flexibility, as it relates to OADs. This derives from the adjective ‘flexibility’, which means the capability of a material, schedule, or personality to bend, adapt or yield.9 Flexibility of an insulin regime or preparation is defined as the ability to inject at variable times, with variable injection–meal time gaps, in a dose frequency and quantity determined by shared decision making. This should require minimal glucose monitoring and health professional consultation, with no compromise on safety, efficiency and tolerability.10
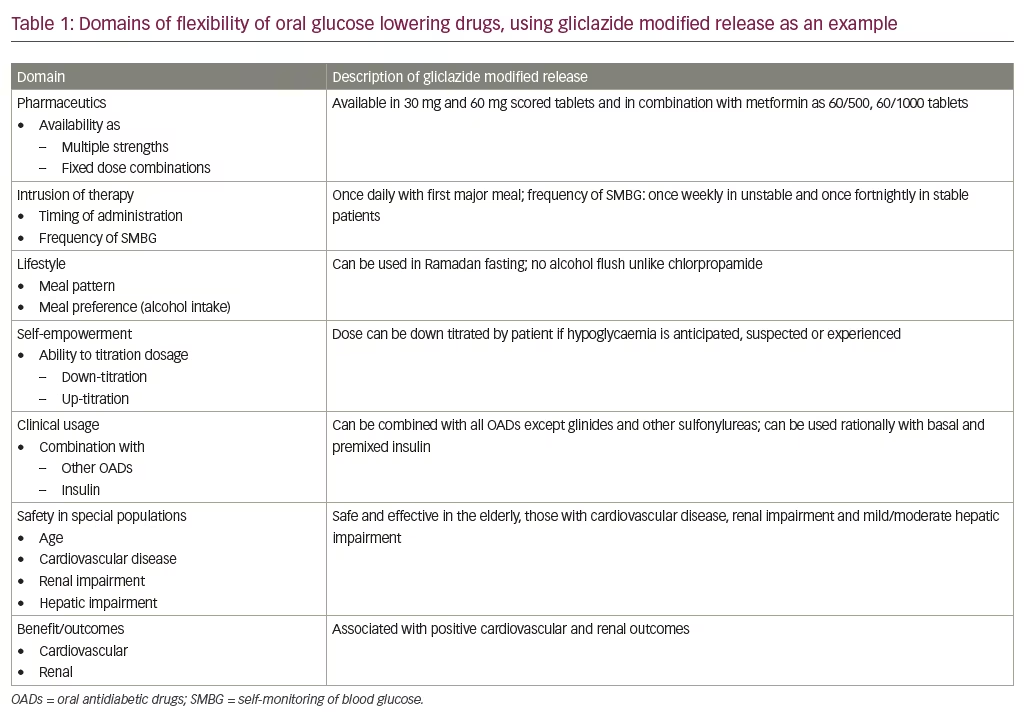
The rubric of flexibility, when applied to insulin regimens and preparations, includes various domains under its umbrella. These include the ability to change time of administration, injection–meal time gaps, site of administration, number of doses, and dosage of units. It also includes the need to adhere to strict dietary and physical activity plan, the recommended frequency of self-monitoring of blood glucose (SMBG), and the requirement for other investigation/concomitant treatment modalities. In diabetes care, the word ‘flexibility’ can be used to qualify treatment targets, treatment regimes, and specific drugs in a variety of doses, dosage frequencies, at times of administration that are convenient, with varying frequency of SMBG and health professional supervision in a wide spectrum of patients, irrespective of their health status.
Gliclazide modified release – the flexi-strong drug
This focus of this editorial is on gliclazide MR, a modern sulfonylurea, as an example of flexibility.
Dosage flexibility and glucose monitoring
Gliclazide MR is available in scored tablets of 30 mg and 60 mg, a range of dosage from 15–120 mg.11 In certain markets, a fixed dose combination (FDC) of gliclazide MR and metformin (60+500 and 60+1000 mg) is available. FDCs containing sulfonylureas reduce cost, offer convenience, and improve patient adherence; therefore, FDCs with varying strengths of sulfonylurea + metformin should be made available, while sulfonylurea + other drugs may be considered.12 These scored tablets provide greater flexibility in dose titration and convenience for patients.
While the initial dose usually depends upon severity of hyperglycaemia and anticipated risk of hypoglycaemia, dose titration is based on the result of SMBG. Modern sulfonylureas offer flexibility in terms of frequency of SMBG. While those with unstable control are suggested once weekly SMBG, persons on stable doses, with good control, can reduce their SMBG frequency to once in two weeks.11
Dose titration
At times a patient may anticipate or suspect hypoglycaemia without having done a glucose measurement.13 In such cases, guidelines encourage the patient to down-titrate the dose themselves. Scored tablets of gliclazide MR facilitate such patient empowerment, as the dose can easily be halved. There may be times when a patient is unsure of her or his appetite or digestion. Such issues occur not only during illnesses with gastrointestinal symptoms but also as a part of a busy lifestyle. Meal timings, quantity and composition may change from day to day in the same individual. As it can be administered with the meal and does not need to be consumed 30 minutes prior to eating; gliclazide MR is a flexible option to choose.14
Fasting and feasting
The flexibility of OADs extends to planned religious fasts such as Ramadan.11 Studies have demonstrated that gliclazide MR can be used safely during Ramadan without a higher risk of hypoglycaemia.15 This advantage is even more pronounced in people who practice intermittent fasting for religious or other purposes. Individuals on a once-daily sulfonylurea should take their medication at Iftar (evening meal), while those on a twice-daily sulfonylurea may shift the morning dose to Iftar and half of the evening dose to Suhur (morning meal).
Patients who follow flexible meal patterns can use flexible preparations of modern sulfonylureas to manage their diabetes. Consensus does not favour the practice of allowing patients to self-titrate their dose upwards. However, in select patients, who are diabetes literate and well-motivated, self-titration can be used to maintain euglycaemia is situations where hyperglycaemia is anticipated or suspected, for example, dining out. Gliclazide MR is not associated with adverse events like the chlorpropamide alcohol flush.16
Gliclazide modified release in combination with other oral antidiabetic drugs
Most patients with type 2 diabetes require combination therapy to manage their condition. Sulfonylureas are flexible in the sense that they can be combined with every class of oral drugs, except glinides. They can also be combined, in a rational manner, with basal, basal-plus and premixed insulin regimens. According to the International Task Force, modern sulfonylureas may be continued with appropriate precaution when basal insulin is initiated.12 They may be continued in the antipodal meal if premixed insulin is initiated once daily. Short-acting sulfonylureas, or glinides, may be continued or added to the third meal with appropriate glucose monitoring if premixed insulin is initiated twice daily.12
Gliclazide modified release across age groups and national boundaries
Another aspect of gliclazide MR’s flexibility is its utility and safety in a wide variety of clinical conditions. It can be administered to elderly patients, as it is associated with low risk of hypoglycaemia. While data on the use of gliclazide MR in children is limited, the rising trend of type 2 diabetes in adolescents may stimulate research in this field. Glibenclamide may be used in the glycaemic control of neonatal diabetes (KCNJ11, ABCC8 gene mutations) and maturity-onset diabetes of the young 3 (MODY 3),11 and gliclazide MR can be studied as an alternative to this.
Gliclazide MR is known to be equally effective across geographic regions of the world.17 Hence, it displays flexibility in use across international borders as well.
Cardiovascular and renal health
Cardiovascular disease (CVD) is the most important complication of diabetes,18 and gliclazide MR demonstrates its flexibility by showing benefit and safety in persons with CVD.3 There are two aspects to this flexibility, firstly, gliclazide MR is safe to use in patients with CVD, unlike glibenclamide, it does not cause ischemic preconditioning.11 It is not associated with risk of increase in stroke or toe amputations,2 and it can therefore be used in patients with, or at risk of, cerebrovascular disease or peripheral arterial disease. There are no reports of association of gliclazide MR with heart failure.
Gliclazide MR is flexible enough to be used across the entire cardio phenotypic spectrum; secondly, gliclazide MR is proven to improve cardiovascular outcomes and leave a positive glycaemic legacy. This is shown by the ADVANCE and ADVANCE ON trials.4,5
Similar flexibility is noted in the renal arena, where early intensive glycaemic control with gliclazide MR is found to be useful, especially in terms of reducing the risk of end stage renal disease.5 Its use is associated with a legacy benefit, as demonstrated in the ADVANCE ON trial.5,17 Moreover, no dose adjustment is needed in chronic kidney disease stages 1–4, but caution should be exercised while using gliclazide MR in patients on dialysis.19 This means that the drug can be used across all reno-phenotypic strata. This flexibility is matched by the ability to improve glomerular health and reduce albuminuria.20
Summary
Gliclazide MR is not only effective, safe and well tolerated,21,22 it is flexible as well. The utility of such a modern sulfonylurea across age groups, clinical presentations and national borders is unique. This multifaceted flexibility, detailed in Table 1, should extend to include flexibility across future decades of diabetes care. The strong evidence base and experience of benefits that gliclazide MR is backed by, will ensure that it remains the drug of choice for uncontrolled type 2 diabetes in future as well.







