Diabetes poses a huge health burden in the US. In the most recent national assessment for 2015, it is estimated that diabetes affects 9.4% of the US population, with costs of diagnosed diabetes reaching $245 billion annually.1 Additionally, it is estimated that 33.9% of US adults are prediabetic and 25.2% of those aged 65 years or older have a diagnoses of diabetes.1 Diabetes is a complex disease requiring on-going medical care and patient self-management to prevent both acute complications and lower the risk of long-term health effects.2 Although glycated hemoglobin (HbA1c) is considered the standard measure of long-term glycemic control in diabetes, it does not provide information regarding incidence of hypoglycemic events or glycemic variability.3 Self-monitoring of blood glucose (SMBG) by patients is an integral part of intensive glycemic treatment, widely believed to improve the control of blood glucose (BG) levels and health outcomes.4
Benefits of self-monitoring of blood glucose
SMBG is an important component of disease management in both type 1 diabetes (T1D) and type 2 diabetes (T2D), allowing patients to evaluate their individual response to therapy and assess whether they are achieving glycemic targets.5,6 Furthermore, SMBG helps patients with diabetes in many ways including: immediately confirming hypoglycemia or hyperglycemia; detecting BG levels that are “out-of-range” so therapeutic adjustments can be made to meet long-term glycemic targets; facilitating patient education as to glycemic responses to medications, food, health state, exercise, or other activities; giving patients more responsibility in the self-management of their disease; and it can help to motivate healthier behaviors in patients.7
While SMBG is crucial to the self-management of insulin-treated diabetes, the benefit of SMBG has not been demonstrated consistently in non-insulin treated T2D.8-12 However, variations in study population, design, and type of SMBG intervention may contribute to the lack of consistent findings. The most recent meta-analysis on SMBG, encompassing 15 randomized clinical trials, and a total of 3,383 patients, concluded that SMBG improved HbA1c levels, both short-term (≤6-month follow-up) and long-term (≥12-month follow-up), in patients with T2D who were not using insulin.13
Factors effecting efficacy in self-monitoring of blood glucose
Studies on the efficacy of SMBG often fail to consider how real-world conditions can affect the performance of monitoring equipment, and that patients vary in their ability to interpret and take action on test results. Inaccurate blood glucose monitoring systems (BGMS) can also lead to adverse health effects, and there is a growing need for post-market surveillance to ensure that these systems routinely meet their labeled accuracy performance in real-world settings.14 Additionally, the benefits of SMBG can be negated by poor patient education on how to interpret the numbers displayed, coupled with a lack of understanding about appropriate interventions following out-of-range BG readings.
Accuracy standards
In 2013, revisions were made to the 2003 International Organization for Standardization (ISO) ISO15197:2003 BGMS accuracy standards (ISO15197:2013) to address the need for more stringent accuracy requirements.15 The requirement states that 95% of BG readings fall within 15 mg/dl (for BG <100 mg/dl), or within 15% (for BG ≥100 mg/dl) of a reference standard. Recent investigations on the accuracy of BG meters have found that many do not meet the new ISO standard.16-20 In 2016, the US Food and Drug Administration (FDA) released a draft guidance document for BGMS that contains additional performance evaluation criteria stating that across the entire claimed measurement range of the SMBG system at least 95% of measurement results shall fall within ±15% and at least 99% within ±20% of the reference measurement values.21
Real-world accuracy
Sources of error in BGMS that can alter BGMS readings, include variations in strip manufacture and storage, environmental factors (e.g., temperature), patient factors (e.g., insufficient hand washing), medical factors (e.g., hematocrit), and pharmacological factors (e.g., interference from certain medications).22,23 Furthermore, during strip manufacture, including variation in material components and manufacturing process can also introduce variation in BG readings. Additionally, BG strips have a typical shelf-life of approximately 21–24 months, which may be further reduced if stored at high temperatures or humidities outside the directions for use. Physical factors such as altitude, humidity, and temperature at time of measurement may also affect BG readings, and certain medications, (acetaminophen being a well-known example), can interfere with BG measurements in electrochemical systems.22,24 Despite these sources of variability, it is estimated that more than 90% of BGMS inaccuracies are caused by incorrect patient use of BG meters.25 For example, in day-to-day use a substantial number of patients do not wash their hands before measuring BG. In addition, handling of high sugar foods such as fruit, can leave traces of glucose on the skin that may confound SMBG results. Lastly, patients do not always store test strips appropriately or pay attention to use-by dates. Since many of these handling problems are due to inadequate patient education, the ease of BGMS use and the quality of the manufacturer’s instructions for use are crucial for reliable and accurate BG measurements.
Patient numeracy
Improved patient outcomes in diabetes rely heavily on the ability of patients to understand instructions from their healthcare professional (HCP) and make appropriate decisions for disease management. Numeracy skills are important for the interpretation of BG readings, calculating carbohydrate intake, and determining adjustments in medication. Numeracy is a significant issue in diabetes self-management,4,26,27 and patients with low numeracy skills are associated with fewer self-management behaviors26 and sub-optimal glycemic outcomes.28 In a recent survey, only 42% of HCPs believed that most of their patients could easily or very easily recognize whether their results were within the correct BG target range when performing SMBG at home.29
Increasing efficacy in blood glucose
monitoring systems
To increase efficacy in SMBG, systems need to be easy to use, provide accurate results, provide ‘in-the-moment’ information, and increase insights for all patients, including those with low numeracy skills. We discuss data on SMBG using OneTouch® BGMS (LifeScan Inc., Wayne, PA, US), outlining newer features designed to promote increased BG testing efficacy.
Glucose test strips
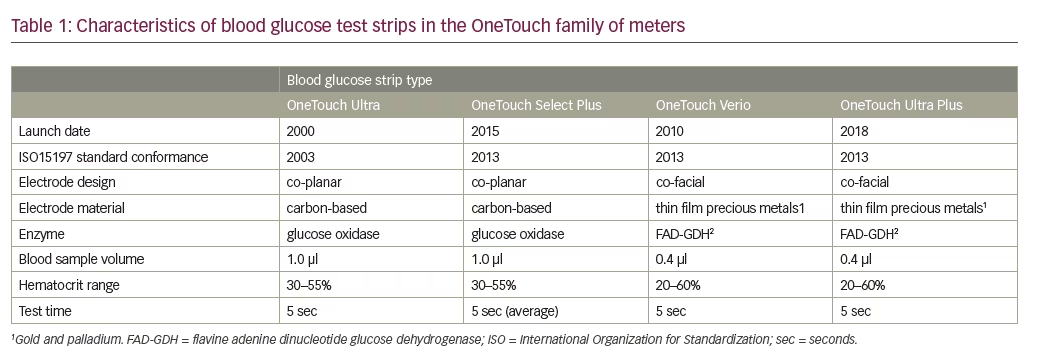
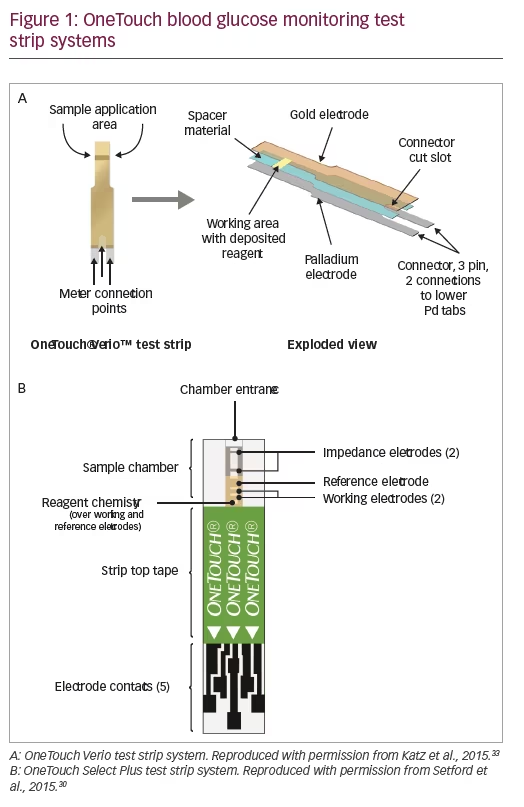
OneTouch BG meters are designed for use with one of four types of electrochemical test strips: OneTouch Ultra, OneTouch Select Plus, OneTouch Verio, and One Touch Ultra Plus (Table 1). OneTouch Ultra test strips use the enzyme glucose oxidase and meet the ISO15197:2003 accuracy requirements. The newer OneTouch Select Plus test strips also use the enzyme glucose oxidase and meet the ISO15197:2013 accuracy requirements.30 OneTouch Verio and OneTouch Ultra Plus test strips also meet ISO15197:2013 requirements, and use gold and palladium based electrodes and the enzyme flavin adenine dinucleotide glucose dehydrogenase (FAD-GDH) to minimize interference from other non-glucose sugars. The electrochemical assay methods and designs used in Select Plus and Verio test strips are shown in Figure 1.
Glucose meters
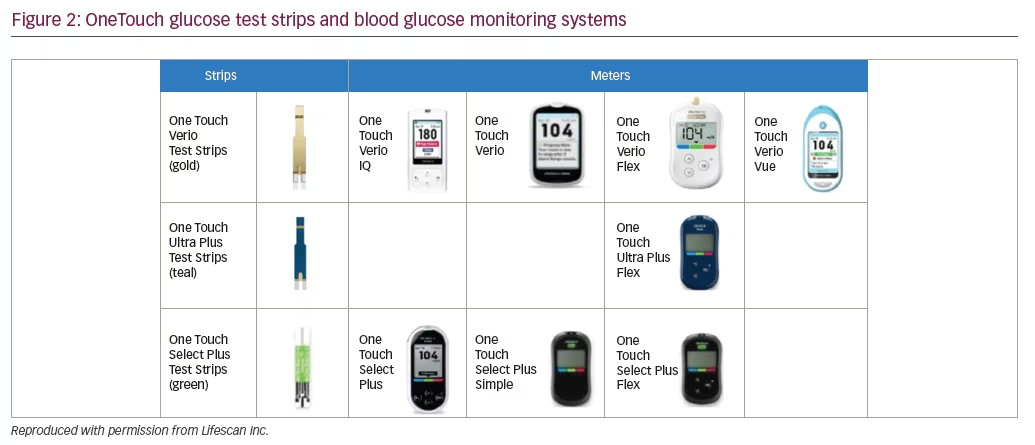
OneTouch test strips are designed for use in the meters shown in Figure 2.
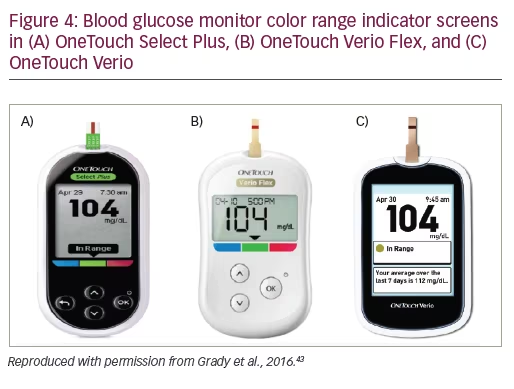
OneTouch meters have proprietary support tools that assist users in interpreting and understanding their BG results plus a variety of features to fit different patient needs. For example, OneTouch Select Plus has a three-color range indicator that can be customized to meet individual glucose targets and a high resolution, easy to read, backlit screen. OneTouch Verio IQ features automatic color-coded messages for high and low patterns, easy one-step meal tagging, a rechargeable battery, and an illuminated test strip port for testing in the dark. OneTouch Verio provides automatic messages with every result, offering feedback and positive reinforcement on how patients are doing managing their BG. OneTouch Verio Flex meters help patients instantly know if their result is in- or out-of-range via a color range indicator and big, easy-to-read numbers, and features capability for BG readings to be transmitted wirelessly to diabetes apps on compatible mobile devices. OneTouch Verio Vue has colorful visuals to help patients easily understand highs and lows over time, an audio testing reminder, and a strip ejector button to satisfy regions where there is sensitivity to handling used BG strips.
Blood glucose monitoring systems accuracy
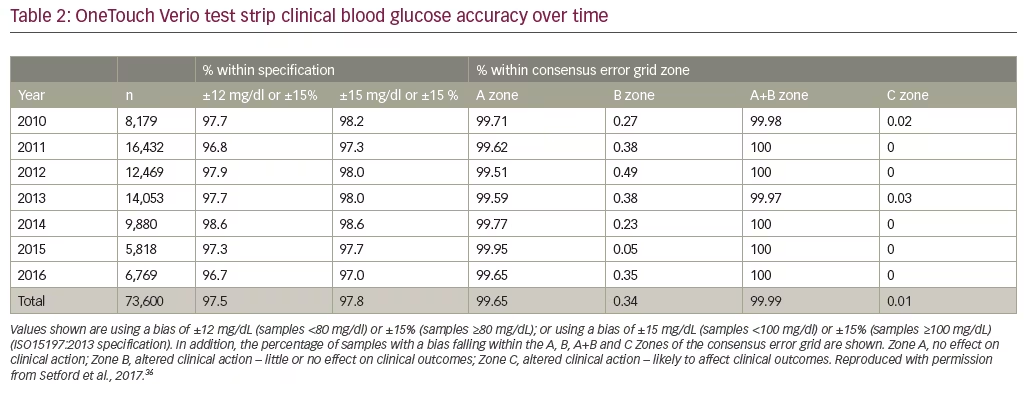
OneTouch Verio test strips have been reported to be accurate over a variety of patient, environmental, and pharmacologic conditions.31-34 Verio test strips are combined with a complex waveform and proprietary algorithm to generate a multi-featured time-current transient response, which can be interrogated to identify and hence minimize the effect of interferents arising from the test sample matrix, thus providing more accurate glucose results. To evaluate the performance of Verio test strips at hypoglycemic BG levels (<70 mg/dL), an analysis was performed using data from seven separate studies (n=700) conducted at two different clinic locations.32 It was found that BGMS utilizing Verio test strips met both the ISO15197:2013 accuracy requirements of at least 95% of measurements being within ±15 mg/dl, or 15% of reference values, and within a stricter ±10 mg/dl or 10% criterion. Similar results were found across all seven studies, importantly with all BGMS supporting the accuracy of the Verio test strip at low BG levels. In another study, hematocrit interference over the labeled range of 20–60% did not affect Verio test strip performance.35 In a study summarizing 7 years of post-market surveillance of Verio strips conducted between 2010–2016 incorporating 73,600 individual glucose results, Verio test strips consistently demonstrated excellent clinical accuracy in accordance with ISO15197:2013, both across the claimed range of BG and hematocrit, and also under the combined
conditions of high and low glucose at hematocrit extremes (Table 2).36
In separate clinical evaluations, OneTouch Verio IQ, OneTouch Verio, and OneTouch VerioVue meters, which each use Verio test strips, all met the ISO15197:2013 accuracy standards for system accuracy and user performance.33 In user performance tests, the patients perform the SMBG test themselves without any assistance from the technician. These BGMS met ISO15197:2013 requirements over a wide range of hematocrit values.
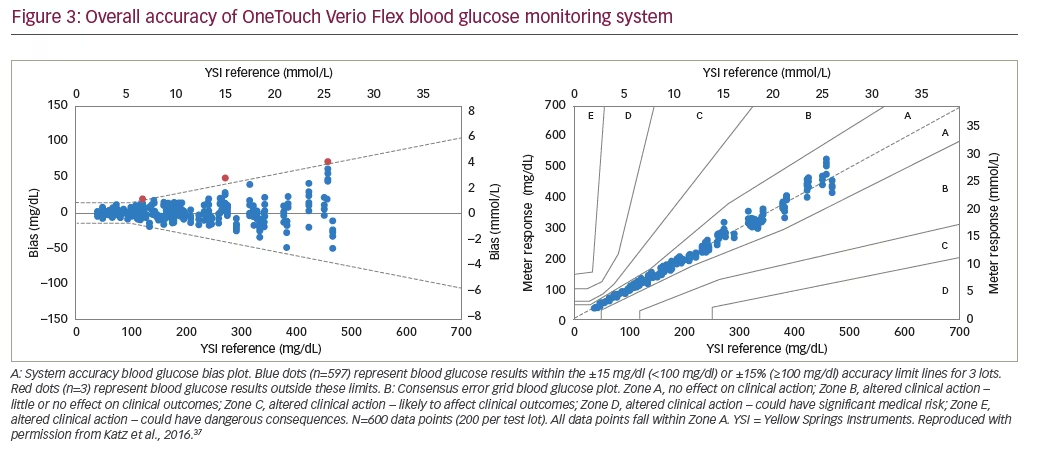
The OneTouch Verio Flex BGMS has been recently introduced into the market. The device uses an optimized algorithm to further improve low glucose accuracy and has Bluetooth® Smart (Bluetooth SIG, Inc., Kirkland, WA,US) functionality to connect with compatible wireless devices. In a clinical evaluation, OneTouch Verio Flex BGMS met user performance and system accuracy ISO15197:2013 requirements (Figure 3A).37 In addition, 100% of the BG measurements were within consensus error grid zones A and B (Figure 3B), indicating no effect (Zone A) or little or no effect (Zone B) on clinical outcomes.38 In a recent independent clinical evaluation by Baumstark et al., three different Verio strip lots in OneTouch Verio Flex meters each fulfilled accuracy requirements of ISO15197:2013, with at least 99.5% of the 200 measurements in each lot within the required limits (±15 mg/dl or ±15%), and 100% of the 600 measurements within consensus error-grid zones A and B.39 Furthermore, 95.5%, 96.5%, and 98.0% of measurement results were within ±10 mg/dl or ±10% of the reference instrument in the three lots.
In clinical studies using capillary blood, system accuracy, and user performance results for the OneTouch Ultra Plus Flex BGMS, each met ISO15197:2013 requirements. A total of 595/600 (99.2%) of system accuracy results and 161/166 (97.0%) of user performance results were within reference standard requirements (data on file). In a laboratory setting using donated venous blood, hematocrit performance and performance requirements for 26 common endogenous and exogenous interference substances also met ISO15197:2013 requirements (data on file).
BGMS which use OneTouch Select Plus test strips also meet all ISO15197:2013 requirements.30 The strip design incorporates additional electrodes that interrogate a key property of the sample (impedance) to estimate the level of hematocrit present within the sample coupled to a proprietary algorithm to provide BG results that meet the requirements of the 2013 ISO standard.30
Meter simplicity/ease-of-use
As mentioned previously, most BGMS inaccuracies result from incorrect patient use of the meters.40 To increase efficacy in SMBG and decrease patient handling errors, BGMS need to be simple and easy to use. Several studies have evaluated patient and/or HCP feedback on the ease-of-use of OneTouch glucose meters. In a study by Katz et al., patient satisfaction with the OneTouch Select Plus Simple meter was evaluated in a home setting.29 As the name suggests, Select Plus Simple is designed as a simple meter with a large display, big easy-to-read numbers and no buttons. A total of 97% of patients strongly agreed or agreed that “the meter is so straight forward, I could use it right out of the box”. In evaluations of the OneTouch Verio Flex meter in patients and HCPs, high levels of satisfaction were also reported.37 Ninety-two percent of HCPs agreed that the meter would be simple and easy for patients to learn. In an evaluation of patient experience with the OneTouch Verio meter, 97% of participants found it easy to use and 99% found the results easy to understand.41
Color range indicators
The ability of patients with diabetes to successfully achieve glycemic control depends, in part, on their ability to identify and interpret glucose results and respond appropriately to abnormal values. A color range indicator is a recent feature in several OneTouch meters to simplify data interpretation and improve ease-of-use. Color range indicators in OneTouch BGMS utilize ColorSure™ Technology, which uses color to indicate whether BG levels are low (blue), in-range (green) or high (red) based on default target glucose ranges or ranges set by the patient or HCP (Figure 4). When patients were surveyed on their use of color in the OneTouch Verio Flex meter, 93% of patients agreed or strongly agreed that color made results simple and quick to understand and helped them easily interpret their BG readings.37
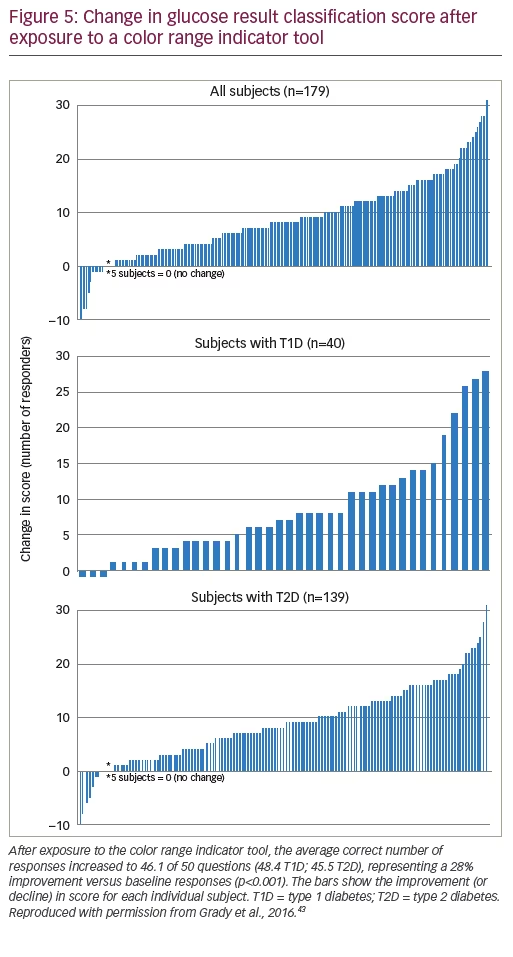
In a study to determine if color could improve patients’ ability to interpret BG values, 59 subjects with T2D were tested on their ability to categorize BG values into low, in-range, and high glycemic ranges.42 After a short interactive session with a meter simulator that used ColorSure technology, the ability of subjects to correctly categorize BG values into recommended ranges was significantly improved, and 90% of patients agreed that ColorSure technology “helps me easily interpret my BG readings”. Another study in 179 subjects with T2D or T1D reported that both groups significantly improved their ability to categorize BG results into correct glycemic ranges after experience with a meter that used color (Figure 5).43 Grady et al. evaluated whether numeracy skills influences decision-making in people with T1D and T2D using a meter with color range indicators.44 Subjects with low numeracy were more likely to take action for high BG results shown with color (61%) than without color (39%). In addition, 68% of all subjects tested agreed or strongly agreed that “ColorSure technology makes it simpler to know when to act compared to a meter without color” and that “color could help them understand when they need to take action along with the BG number”.
The use of color range indictors has also been shown to assist in the improvement of patients’ metabolic control and diabetes management. In a recent study in 163 subjects with T1 and T2, HbA1c decreased by 0.4% after 12 weeks in subjects who were switched to OneTouch Verio and Verio Flex meters (with color range indicators), compared to a control group who continued to use their own meter without color indicators (p=0.01) (data on file).
Pattern recognition
To determine the best course of glycemic management, patients have traditionally recorded their BG measurements in a paper logbook for review by their HCP, a practice that is time consuming for both patient and HCP. Furthermore, errors may be introduced and fear of negative feedback can cause some patients to inaccurately represent their data. Because office visits with a HCP are often short, less time spent on logbook review could allow better individual disease management. Some BGMS contain algorithms that instantly recognize high and low BG patterns or trends and alert the users in real-time. The use of pattern recognition tools can aid both the HCP in evaluating patient glycemic history and the patient by providing ‘real time’ messaging which may prevent untoward events, such as severe hypoglycemia.45
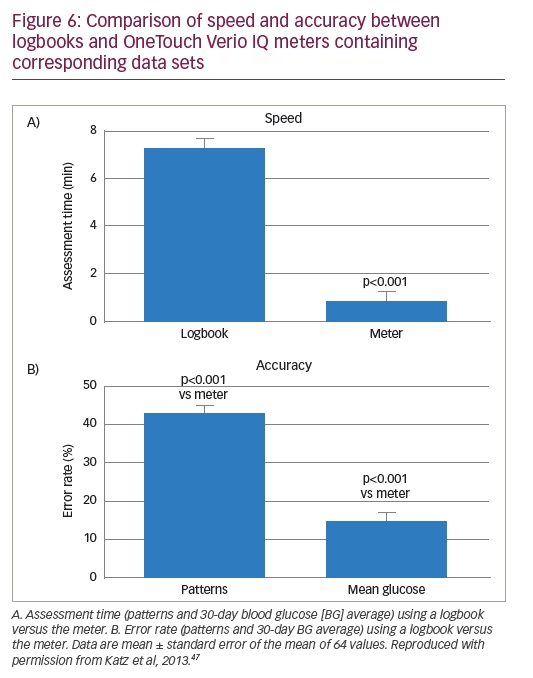
In a study that evaluated glucose pattern recognition tools, 71.3% of study subjects indicated that they preferred to use a meter with pattern messages.46 OneTouch Verio IQ incorporates PatternAlert™ (LifeScan Inc., Wayne, PA, US) technology, which analyses BG readings in the meter’s memory, identifies glycemic patterns, and notifies the user with a message in ‘real time’. In a study by Katz et al., 64 HCPs were asked to assess glycemic patterns and estimate BG averages in handwritten logbooks and in data from the OneTouch Verio IQ meter.47 Review of data sets from this meter was associated with faster and more accurate pattern analysis when compared with handwritten logbooks, with pattern review taking an average time of 7.3 min for handwritten logbooks versus 0.9 min using the meter (Figure 6A). The total pattern recognition error rate of 43% when using a logbook was also significantly reduced by using the meter to identify patterns (Figure 6B).
When the recognition of BG patterns in simulated logbooks was compared to using the OneTouch Verio meter, it was found that use of this meter resulted in four times faster recognition of BG patterns by HCP.41 When surveyed on the value of this BGMS, 97% of HCPs agreed that the range indicator, automatic pattern messaging, and progress notes features would make interpreting the results much easier for patients with diabetes, compared with a traditional meter. A survey of patients using OneTouch Verio meter for one week found that 93% agreed that the meter “helps me to better understand my results so I can make better decisions”. Importantly, a 6-month observational study of 193 subjects using OneTouch Verio meters resulted in significant improvements in glycemic control, as demonstrated by a decrease in mean HbA1c levels from 8.7% at baseline to 8.1% after 3 months, and 7.9% at 6 months (both p<0.0001).48 Additionally, based on the validated Diabetes Self-Management Questionnaire (DSMQ) instrument, patients’ general perception improved significantly by the end of the study. Both patients and diabetes educators agreed that color-coded indicator and pattern recognition in the meter assisted patients in their diabetes management.
Connectivity to diabetes management apps
Cloud-connected diabetes applications offer the potential for flexible patient care, allowing HCPs to monitor patient progress and deliver care remotely. OneTouch Reveal® is a cloud-based application with both web and mobile versions that collects BG data and provides analytics to better inform treatment choices and lifestyle decisions. The OneTouch Reveal application summarizes BG or insulin data, and presents trends and BG patterns using a color-coded display. It also delivers online educational tips and enables sharing of patient data with their HCP in ‘real time’.
In a study exploring patient experiences using the web-based Reveal application in combination with OneTouch Verio meters, subjects uploaded BG results to Reveal over the course of 12 weeks.49 HCP telephone consultations occurred at 4 and 8 weeks following remote review by the HCP of the BG data. Use of OneTouch Verio meters in combination with the OneTouch Reveal web-based app improved glycemic control with a mean HbA1c decrease of 0.4% (p<0.001). A total of 83% of patients agreed or strongly agreed, that “Reveal helped them to consider the choices they made”, and that “Reveal helped them to see the big picture and motivated them to stick to their plan”.
OneTouch Verio Flex meters have Bluetooth® technology, allowing it to send BG data off-meter to software/apps on compatible wireless devices such as a smart phone. To determine HCP satisfaction with the OneTouch Reveal mobile app, a diverse group of HCPs were surveyed for their opinion of the OneTouch Verio Flex meter alone and in combination with OneTouch Reveal.37 Eighty-eight percent of HCPs agreed that the mobile application could help reinforce treatment recommendations with patients, and 91% agreed that along with treatment recommendations, combined use of the meter and mobile app could help patient engagement between HCP visits. In a 24-week outcome study in patients with T1 and T2D, patient experiences and changes in glycemic control were assessed using OneTouch Verio Flex with wireless connectivity to OneTouch Reveal.50 Patients were randomly assigned to use the glucose meter alone (n=66) or the meter plus the OneTouch Reveal app (n=62) and clinical measures and self-reported outcomes were assessed at baseline, week 12 and week 24. Patients in the meter plus app group also received text messages from an HCP every 2 weeks based on the app report. At 12 and 24 weeks, significant HbA1c reductions of 0.78% and 0.67%, respectively, were observed compared with baseline HbA1c of 8.9% (p<0.001). A total of 95% of patients agreed “the colorful visuals and pattern messages in the Reveal app told them when they were doing well and when they needed to pay more attention”. A large proportion of subjects (88%) thought that ColorSure technology in combination with the Reveal app could help them stay on track between visits to their HCP.
Conclusions
In patients with diabetes, SMBG is an important tool to determine whether they are achieving glycemic control and to evaluate their individual response to disease management strategies. Real-world conditions can affect SMBG efficacy, and the ability of patients to understand and act on their BG results is highly variable. For SMBG to be effective, BGMS need to be accurate, simple and easy to use. Similarly, the improvement of disease management relies on increasing patient insight and giving patients access to ‘in-the-moment’ information, including in those patients with low numeracy skills. Newer BGMS contain features including color range indicators, pattern recognition, and diabetes app connectivity, which seek to improve SMBG interpretation and increase efficacy of testing. ColorSure Technology has been demonstrated to improve patients’ ability to interpret BG readings and increase the likelihood that patients will act on their BG results. Similarly, the use of pattern recognition to identify high and low glycemic patterns and notify the user with a message in ‘real time’, may help patients with self-management behaviors and potentially avert significant hypoglycemia and hyperglycemia. This technology is also highly beneficial to HCPs during BG data review and interpretation, resulting in much faster recognition of BG patterns when compared with logbooks. Finally, the ability of HCPs to have real-time visibility of patient data within diabetes management apps has the potential to improve personalized disease management.
Given the struggles that many patients have with ongoing adherence, motivation, health literacy and/or numeracy, the newer BGMS features described in this article represent important tools which may help HCPs better communicate with their patients on how to both understand their BG data and encourage appropriate self-management from their patients. The future use of these technologies for ‘real-time’ motivational, behavioral, and educational coaching, will potentially result in better health outcomes for patients who poorly manage their diabetes.







