Gestational diabetes mellitus (GDM) has traditionally been defined as carbohydrate intolerance, with its onset or first recognition in pregnancy.1,2 The definition, screening and diagnosis of GDM continue to be subjects of controversy.3 Recent guidelines have emphasised the importance of the definition of GDM and its distinction from overt diabetes mellitus in pregnancy, because this definition affects the management of the condition during and after pregnancy.4 Even a mild degree of hyperglycaemia is associated with a linearly increasing rate of adverse pregnancy outcomes, with no inflection point for risk.5,6 Therefore, a diagnostic cut-off value can be considered arbitrary, and this has created confusion about how best to screen for and diagnose GDM.3,7
Based on the Hyperglycemia and Adverse Pregnancy Outcomes study, the International Association of Diabetes and Pregnancy Study Group has suggested screening all pregnant women without known pre-existing diabetes or GDM at 24–28 weeks of gestation. The recommended test for screening and diagnosis is the 2-hour 75 g oral glucose tolerance test (OGTT).8 These recommendations have also been adopted by the World Health Organization (WHO).4 In contrast, the American College of Obstetricians and Gynecologists (ACOG) suggests that screening should be based on a 50 g OGTT, followed by a 3-hour 100 g OGTT, for the diagnosis.9 According to the European Association for the Study of Diabetes, the diagnosis of GDM should be based on a 75 g OGTT.10,11 In the UK, the National Institute for Health and Care Excellence (NICE) guideline suggests that previously healthy pregnant women should be screened only if certain risk
factors are present.12 This approach differs critically from the other well-established guidelines discussed above.
In Sweden, the National Board of Health (Socialstyrelsen) adopted the new WHO and International Association of Diabetes and Pregnancy Study Groups thresholds for the diagnosis of GDM in 2015, but leaves it up to local health authorities to specify the strategy for screening.8,11 However, these guidelines do not provide a national recommendation for screening for GDM because the evidence for different screening strategies was found to be inadequate.11 The main screening strategy used in Sweden is based on repeated measurements of capillary random plasma glucose (RPG) and the assessment of risk factors for the development of GDM.13,14 When RPG ≥9.0 mmol/l and/or risk factors for GDM are present, the one-step, 2-hour 75 g OGTT is recommended in gestational weeks 28–32.15,16
There are several predisposing factors for GDM, including maternal body mass index (BMI) and maternal age.17,18 Selective screening may be cost-effective, but risk-factor-based screening has been shown to miss a substantial number of women with GDM.19,20 The use of other strategies, such as RPG, is also controversial. RPG is a quick, simple and inexpensive test that measures plasma glucose at a random time, regardless of the time of the last meal. There are few studies on RPG as a screening test for GDM. Although the results are not conclusive they suggested that the RPG test may result in high false positivity and false negativity.21,22 The latest NICE guideline did not recommend using RPG to assess the risk of developing GDM.12 A previous systematic review found limited evidence to support the use of RPG in screening.23 In contrast, a recent study suggested that RPG predicts GDM better than maternal age or BMI.24
We hypothesised that women with GDM would have elevated blood glucose levels before its diagnosis. The aim of this study was to assess the performance of RPG in gestational weeks 23–28, alone and/or in combination with maternal BMI and age, in identifying women who will develop GDM.
Material and methods
In a retrospective cohort study, the data for all pregnant women who gave birth in Västernorrland County, Sweden, over a 2-year period (1 January 2015 to 31 December 2016) were obtained. This study was approved by the Regional Ethical Review Board in Umeå, Sweden.
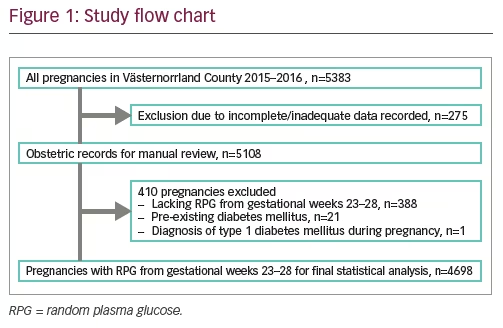
The Swedish electronic medical record database for prenatal care and childbirth, Obstetrix (Siemens Corporation, Upplands Väsby, Sweden), was searched for women meeting the criteria defined below. In Obstetrix, each pregnancy is followed in a logical and structured manner, from enrolment in a prenatal healthcare centre to the arrival at the maternity unit and the time of delivery. Data for pregnancies fulfilling our criteria were compiled into an Excel file. For each pregnancy, the records were searched manually for the RPG value at gestational weeks 23–28, and whether or not each woman developed GDM was recorded. Women were excluded for the following reasons: incomplete data recorded, lack of an RPG test in gestational weeks 23–28, pre-existing diabetes mellitus and/or other metabolic disease, or a diagnosis of diabetes other than GDM during pregnancy. Maternal hypothyroidism was not considered an exclusion criterion.
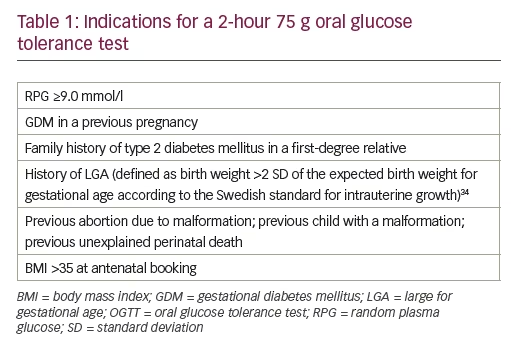
The main screening strategy for GDM was based on repeated capillary RPG measurements made four to six times during pregnancy. The risk factors for GDM that indicated a 2-hour 75 g OGTT, according to regional screening practices, are presented in Table 1. The main diagnostic criterion for GDM was based on the one-step 2-hour 75 g OGTT. After 2 hours, a capillary RPG of ≥10.0 mmol/l (considered equivalent to venous blood glucose ≥9.0 mmol/l) was considered pathological and defined as GDM. Fasting capillary glucose values were not considered as a diagnostic criterion for GDM.
IBM SPSS Statistics version 24.0 (IBM Corp., Armonk, New York, USA) was used for all statistical analyses. The ability of capillary RPG, maternal BMI, and age to predict GDM was tested with receiver-operating characteristic (ROC) curves. The variables presented in Table 2 were compared between the two groups of women: with and without GDM, using an independent-samples t test and χ2/Fischer’s tests for continuous and categorical variables, respectively. In this study, the sample size calculations were based on the diagnostic test accuracy under different conditions and test results. The required sample size was calculated to be n=4,800 based on an estimated prevalence of GDM by the latest national statistics in southern Sweden25 (2.6%), a margin error of 0.07 (confidence width = 0.14) with 95% confidence, and an estimated sensitivity of 80%.
Results
Data for 5,383 pregnancies were initially obtained. After the exclusion criteria were applied, a total of 4,698 pregnancies qualified for the final statistical analysis (see flow-chart, Figure 1).
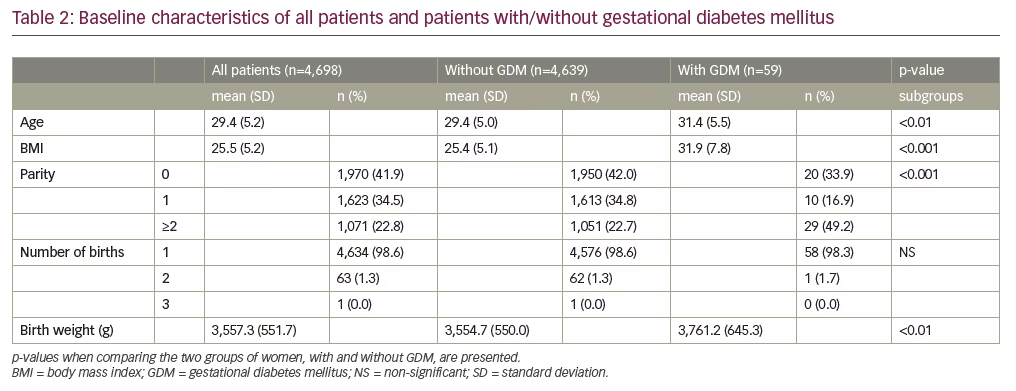
Women without pre-existing diabetes who received a diagnosis of diabetes during pregnancy were considered to have GDM.11 However, the persistence of diabetes was not followed postpartum. The number of pregnancies associated with GDM was 59 (out of 943 OGTT), equivalent to a prevalence of 1.3% in the study population.
The baseline characteristics of all pregnancies and subgroups, according to the presence or absence of GDM, are shown in Table 2. Women with GDM were older (p<0.01) and had a higher BMI (p<0.001) than those without GDM. Parity (p<0.001) and foetal birth weight (p<0.01) were higher among the women with GDM. There was no statistically significant association between GDM and the number of births.
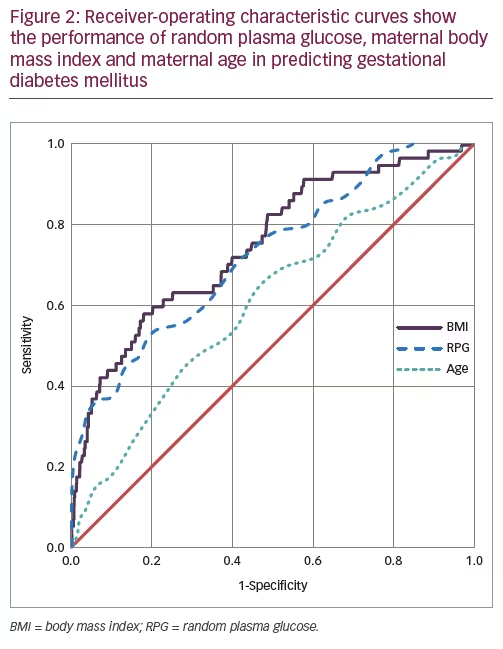
The abilities of RPG, maternal BMI, and age to predict GDM were tested with ROC curves (Figure 2). The thresholds that generated the best overall performance for each variable and different combinations of variables were assessed. The threshold/combined thresholds generating the maximum sum of sensitivity and specificity were considered to show the best overall performance.
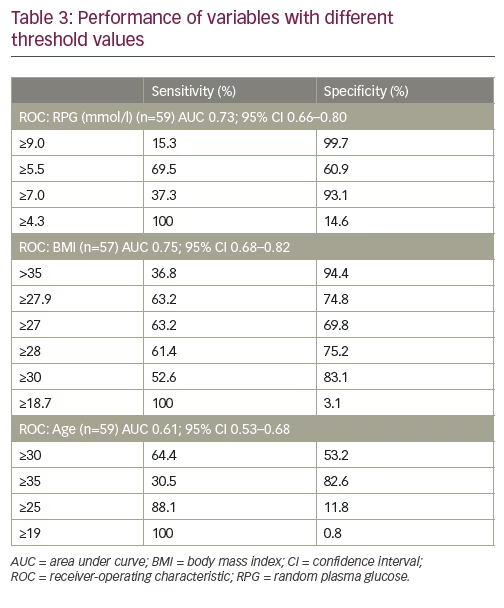
RPG predicted GDM fairly well (n=59 with RPG; area under the curve [AUC] 0.73; 95% confidence interval [CI] 0.66–0.80). The threshold value of RPG ≥9.0 mmol/l showed a sensitivity of 15.3% and a specificity of 99.7%. A cut-off value of RPG ≥7.0 mmol/l showed greater sensitivity (37.3%) and a specificity of 93.1%. To achieve 100% sensitivity, the cut-off for RPG had to be set at RPG ≥4.3 mmol/l, which would have identified all cases of GDM, but the specificity would have decreased to 14.6%. The best overall performance of sensitivity and specificity was obtained with RPG ≥5.5 mmol/l. Using this cut-off value, RPG predicted GDM with a sensitivity of 69.5% and a specificity of 60.9% (Table 3).
BMI predicted GDM with similar AUC values, as did RPG (n=57 with BMI; AUC 0.75; 95% CI 0.68–0.82). The threshold value of BMI >35 had a sensitivity of 36.8% and a specificity of 94.3%. The overall best performance of sensitivity and specificity was achieved with a threshold of BMI ≥27.9, with 63.2% sensitivity and 74.8% specificity (Table 3).
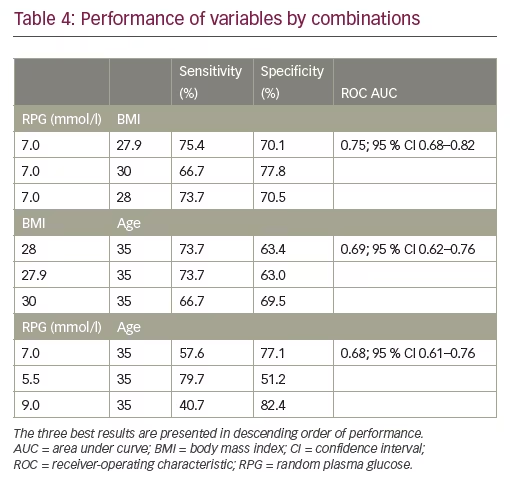
Maternal age performed poorly as a screening factor for GDM (n=59 with age; AUC 0.61; 95% CI 0.53–0.68) (Table 3). The performance of different combinations of RPG, maternal BMI, and age in predicting GDM was controlled. The combination of RPG ≥7 and BMI ≥27.9 showed the best performance, with a sensitivity of 75.4% and a specificity of 70.1% (ROC AUC 0.75). Generally, compared with the performance of individual factors, increased sensitivity was obtained by combining the factors, but at the cost of lower specificity and lower ROC AUC (Table 4).
Discussion
There is no consensus regarding optimal standard for screening and diagnosis of GDM. The results of this study show that a capillary RPG at gestational weeks 23–28 has only a fair performance in identifying women who develop GDM. Maternal BMI as a screening factor showed a slightly better performance, whereas the performance of maternal age was poor. On the other side, combining RPG and BMI increases the overall performance and the sensitivity, while the specificity of the test decreases.
RPG assessment is a convenient screening method that can be applied at a prenatal visit. Some studies suggested that the RPG measurement in early period of gestation may show negative results with a low positive predictive value (6.7%).21 The changes in maternal carbohydrate metabolism and the increasing insulin resistance are well established by 24 weeks of gestation and GDM is most likely to develop after this time as the capacity of the pancreas to adapt to the increased need of insulin is impaired, especially in women with predisposing factors.3 It is thus reasonable to assume that elevated blood glucose levels can, at the earliest, be detected around and after gestational week 24. The benefits of earlier screening are unclear and the US Preventive Services Task Force therefore recommends screening for GDM after gestational week 24.26 Screening of GDM in Sweden is based on repeated capillary RPG measurements during pregnancy, and an RPG should be measured around gestational week 24–25.14 However, the recommendation is not definite and slight variation in sampling time may occur. As a consequence, some women might have an RPG value at gestational week 23. We thus included women with RPG values registered at gestational weeks 23–28.
Previous studies on the performance of random glucose measurement in screening for GDM show considerable heterogeneity in study design, diagnostic criteria, definitions and methods. In a systematic review, van Leeuwen et al. attempted to calculate a summary estimate of the performance of a random blood glucose test in screening for GDM. However, the author declared that the available evidence on the accuracy of a random glucose test in screening for GDM is limited, a single random glucose measurement was concluded to be inadequate to screen for GDM.23 Jowett et al. compared the performance of repeated measures of random venous blood glucose at gestational weeks 27–31 with a 2-hour 75 g OGTT. In the same line as in our study, they showed a low sensitivity and a high specificity for random blood glucose test; however, the performance of the test varied depending on the timing of the sample collection. On the other hand, the majority of women with impaired glucose tolerance would be missed, therefore the author concluded that random blood glucose measurement is not a sufficiently sensitive method for detecting GDM.27 In another study, the performance of random blood glucose test in screening for GDM was compared with a 50 g OGTT at 24–28 weeks of gestation. The 50 g OGTT is the most widely accepted method to screen GDM in north America.26Their results showed better performance of the 50 g OGTT, while a study by Mathai et al.22 suggested that neither RPG nor the glucose challenge test is a useful screening method for GDM.28 In all the above-mentioned studies, a venous blood glucose test was used instead of a capillary RPG and tests performed during different gestational weeks, which makes the comparison between studies difficult.
In our study, RPG measured via capillary blood sampling, and it can be accounted as a limitation of this study. According to international consensus, the diagnosis of diabetes should be based on venous sampling.29 Venous and capillary values are considered to reflect different phases in the body’s glucose metabolism, which may vary from one individual to another, which means they are not directly interchangeable. Capillary values are believed to reflect glucose absorbed from the intestine and venous host elimination and the body’s need and ability to metabolise glucose.30 Venous sampling is complicated and time consuming, as well as being more uncomfortable for the patient. Capillary analysis has the advantage of giving an immediate response and simplifies handling compared with the samples being sent to the laboratory for analysis; however, measurement safety is usually worse in capillary test apparatus compared with the central laboratory instruments.31
Another limitation of this study is that we have not taken into account the ethnicity factor. Ethnicity has long been described as a major risk factor for the development of GDM.
In contrast to our results, a recent study by Meek et al. showed that RPG have a good performance in screening for GDM (AUC 0.81; 95 % CI 0.80–0.83), and RPG even performed better than predisposing factors for GDM (maternal age and BMI) when used as a screening test for GDM,24 however, similar to our results, they also found that combining RPG and BMI, or BMI and age improved the sensitivity. The difference between studies can be explained by the fact that in the study by Meek et al., the study group consisted of pregnant women at early gestational age (12–16 weeks) while, in our study, data collected were from among pregnant women at later gestational ages (23–28 weeks).
The threshold value of RPG ≥9.0 mmol/l that has been used in this study to determine if a further OGTT is needed, was able to identify 15.3% (n=9) of women with GDM. On the other hand, the number of healthy women with an RPG value above this cut-off value was very low (0.3%, n=12), therefore, this threshold value (≥9.0 mmol/l) may fit better in women with no additional predisposing factors for GDM. On the other hand, lower threshold (<9.0 mmol/l) would identify more cases of GDM, but the number of false positive samples would be increased and more healthy women would need to undergo an OGTT.
In Sweden, the main screening strategy for GDM is based on repeated capillary random blood glucose and assessment of risk factors for developing GDM;14,15 however, this selective screening strategy is suggested to be insufficient when compared to an OGTT.20,13 Our results also confirm that selective screening is insufficient because neither of the variables we controlled (commonly used in selective screening) could show compelling performance alone or combined. In countries with growing epidemics of obesity and diabetes, many women in most populations will have some risk factors for GDM depending on the criteria used. Thus, the implementation of risk-factor-based screening will require most women to be tested. On the other hand, OGTT screening misses some patients with increasing carbohydrate intolerance that do not meet the current criteria for the diagnosis of GDM because of the rate of unavoidable no-shows.32,33 The Hyperglycemia and Adverse Pregnancy Outcomes study demonstrates that maternal hyperglycaemia, even at a level below that diagnostic of GDM, leads to increased morbidity during pregnancy and is associated with adverse pregnancy outcomes.5 It is open to speculation how the screening by a combination of risk factors and an OGTT may change the number of missed diagnoses.
Based on national statistics in Sweden, the prevalence of GDM is highest in Southern Sweden, where universal screening with an OGTT has been practiced since 1995.25 The difference in prevalence numbers for GDM in this study (1.3% versus 2.6% in southern Sweden) is presumably due to the screening practice and indicates that there could be a substantial underdiagnoses of GDM in the remaining parts of Sweden. It appears that the diagnostic accuracy for GDM is higher for an OGTT than a capillary RPG and, therefore, offering an OGTT with venous sampling to all pregnant women should be the most reliable screening strategy. Although the recommendation to undertake OGTT in every woman to screen for GDM would have a defined cost, there might be a long-term economic advantage in the early diagnosis and management of GDM, as women at risk of developing diabetes in the future could be detected early on, with the possibility for primary preventive actions to be taken.
In conclusion, it is known that untreated GDM leads to increased maternal and perinatal complications, but the screening methods for diagnosis remain controversial. Capillary RPG is a convenient and inexpensive test for screening for GDM, but our results suggest that sensitivity is poor. Future research on screening methods for GDM should be conducted in a prospective design.







