Ketogenic diet (KD) is defined as a high-fat, low-carbohydrate diet, with adequate protein content, which makes the body utilize fat, rather than carbohydrate, as a preferred energy substrate. In a carbohydrate-replete person, this substrate is converted to glucose, which is used as a fuel by all organs, including the brain. In carbohydrate depletion, ketogenesis is activated in the liver, which breaks fat into fatty acids and ketone bodies. These ketone bodies are able to cross the blood–brain barrier and provide energy to the brain. Ketones can also be utilized by other organ systems as an efficient energy source.
Development
The term ‘ketogenic diet’ was first used by Russel Wilder in 1923 to describe a high-fat, low-carbohydrate diet that produced ketonemia. He used it as an alternative to fasting (then in vogue as a therapeutic option) for the management of epilepsy.1
Various forms of KD are now used in clinical practice. The amount of carbohydrates permitted varies from 20 to 50 g/day. This depends upon personal metabolic and weight loss goals, upon the planned duration of KD, and upon individual health status. The classic therapeutic KD, initially created for the management of childhood seizures, has a 4:1 ratio of fats to combined protein and carbohydrates. A medium-chain triglyceride (MCT) variant (the MCT KD) utilized to more ketogenic MCT (present in coconut oil) to provide half of all consumed calories. The Atkins diet—popular in lay literature—and the low glycemic index diet are less restrictive variants of KD. Fluid restriction is not advocated in modern KD, due to the risk of constipation and nephrolithiasis. Vegetarian and vegan KD are also available.2
Clinical approach
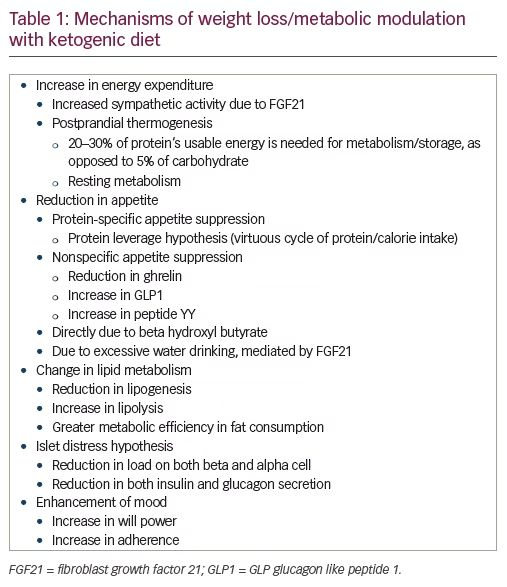
KD is a therapeutic intervention for diabetes and obesity.3,4 Though nonpharmacological in nature, it has a well-delineated multifactorial mechanism of action (see Table 1). Thus, it should be approached as a therapeutic intervention, rather than a universal suggestion. KD stands apart from other weight-lowering options in certain ways. Most drugs that increase energy expenditure also increase food intake, and thus create a net effect of weight maintenance, rather than loss.5 Drugs that reduce food intake, such as phentermine, liraglutide, phentermine/topiramate, and buproion/naltrexone, do not reduce energy expenditure. Maintenance of a lowered body weight leads to compensatory changes in energy expenditure, which limit the potential for change. These compensatory changes may account for the poor long-term efficacy of treatments for obesity.6
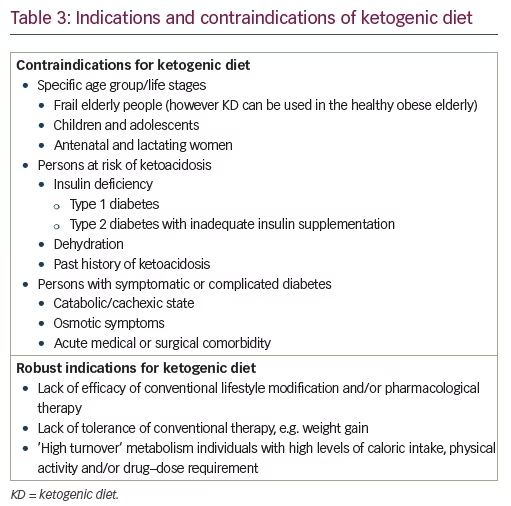
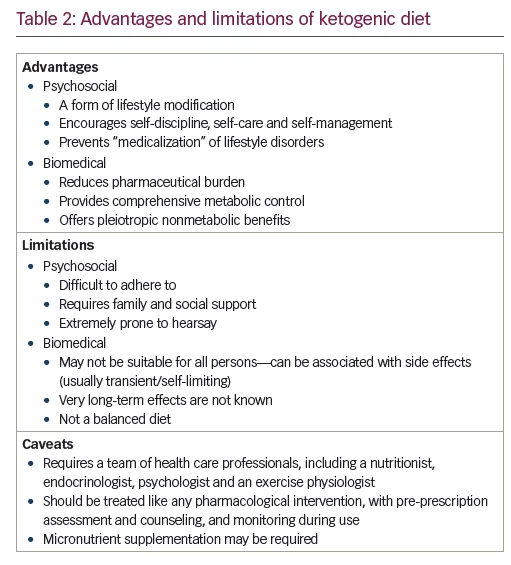
The efficacy, safety, and tolerability of KD has been studied in preclinical and clinical settings, including randomized controlled trials. The limitations of KD, such as contraindications, limitations of sustainability, issues in adherence, and possible adverse events, are known as well (see Tables 2 and 3). In all these matters, KD appears similar to drug therapy and should be studied as such.
Biochemistry
The absence of adequate dietary carbohydrates leads to a drastic reduction in insulin levels, which leads to reduced lipogenesis and increased lipolysis, both of which reduce fat accumulation. Continued carbohydrate starvation leads to the inability of usual metabolic pathways (the Krebs cycle) to provide adequate glucose supply to the central nervous system (CNS).7
To maintain CNS health, the liver kick-starts a process of ketogenesis, producing acetoacetate, which gets converted to β-hydroxybutyrate, the predominant circulating ketone body. High levels of acetoacetate cannot be metabolized fast enough by the skeletal muscles and myocardium. Hence both acetoacetate and β-hydroxy butyrate rise in the circulation, leading to ketonemia and ketonuria. Excretion of acetone, a volatile ketone body, through the lungs, causes the sickly-sweet odor of ketosis. Once ketone bodies achieve a blood concentration similar to that of glucose (4 mmol/l; 80 mg% glucose), they can be transported preferentially, across the blood brain barrier, into the brain. Here, they are a more efficient source of energy.8
Mechanism of action
KD acts by inducing a state of physiological ketosis. This is achieved by consuming minimal carbohydrates, thus creating a state of carbohydrate starvation. As carbohydrate substrate is minimized, the insulin requirement comes down. This leads to resolution of insulin resistance and reduction in insulin secretion, with a concomitant fall in glucagon production (the islet de-stress hypothesis) (personal communication). Caloric and nutrient intake is maintained through fats and protein. Fats are preferentially utilized to produce energy for the body and are burnt up.
KD’s weight reduction is thought to be mediated by a reduction in hunger (general and protein specific) and an increase in energy expenditure (resting and postprandial).9,10 Lipogenesis is reduced; lipolysis increased. Gluconeogenesis, which occurs in KD, has an increased metabolic cost and leads to use of body fat stores11,12 (see Table 1). KD-induced weight loss is accompanied by a mitigation in increase of circulating ghrelin. This helps avoid hunger and cravings and contribute to maintenance of weight loss.13
Pre-intervention assessment
KD must be prescribed and monitored by qualified health care professionals, who are experienced in KD use.14,15,16 Coordinated team work, between an endocrinologist, nutritionist, psychologist, and an exercise physiologist, is required to achieve optimal results. The principles, expected efficacy and possible side effects17 must be understood. A biopsychosocial assessment and counseling are mandatory for every patient. Indications and contraindications of KD must be assessed prior to start. Ample information must be shared with patients, so that they have a fair idea of what to expect on a KD (see Table 2).8,15 This discussion should cover both expected benefits and risks, as well as responsibilities of the patient and her/his family. KD should be started only in motivated patients. Clarity regarding the purpose and aim of KD, and its expected duration, must be achieved by shared and informed decision making and must be documented.
Robust indications
The contraindications and indication for following KD are listed in Table 3.18 Though evidence is tempting19 one cannot propagate KD as a primordial or primary preventive therapy. However, KD is certainly recommended for secondary prevention in persons with obesity or type 2 diabetes who do not respond to conventional dietary and pharmacological measures for weight loss, hunger control, or glycemic management.
Monitoring
Once KD has been instituted, regular clinical and biochemical monitoring, are required. This should be coupled with medical and psychological support as when necessary. The frequency and intensity of monitoring and support may decrease as the patient gains confidence and takes charge of her/his diet, lifestyle, and health.2 Troubleshooting may be required when sudden changes in lifestyle and health status are anticipated, suspected, or experienced. These may include conditions such as travelling, festivals and fasts, acute undercurrent illness, and planned or emergency surgery. At times, temporary discontinuation of KD may be necessary till an acute episode of illness is resolved.
Persons on glucose-lowering, antihypertensive, or lipid-lowering therapy may need change in dosage and/or in therapeutic strategies.20 The first few weeks of ketoadaptation may be accompanied by fluctuations in glycemia and blood pressure, which should be preempted and managed as required. Ketoversion (shift from nonketonuria to ketonuria) may be associated with transient rise in blood pressure.
Side effects
The side effects of KD include those directly related to KD, such as transient ketoflu, and those related to weight loss in general. Shifting to KD involves a brief (days to weeks) period of keto-adaptation, during which the body transitions from carbohydrate- to fat-based energy utilization.17 This period may be marked by ‘ketoflu’, with symptoms such as fatigue, lethargy, and headache. We have noted that the phase of ketoversion (shift from aketonuria to ketonuria) may not necessarily overlap the duration of ketoadaptation, which is marked by ketoflu (personal observation).
Adverse events, in the short term, include constipation, low-grade acidosis, hypoglycemia, and dyslipidemia.15,17,21 Constipation can be prevented by adequate fluid intake while dyslipidemia should prompt a shift to a less KD, with a lower fat:carbohydrate/protein ratio. Hypoglycemia should be anticipated and prevented by proactive down titration of glucose-lowering medication. A similar strategy should be employed with antihypertensive therapy.
Dehydration and dyselectrolytemia are other side effects of KD that must be prevented and managed. These can result in muscle cramps and arrhythmias.17,21 Supplementation with electrolytes, including magnesium, and multivitamins, helps minimize these adverse events. KD has neurotropic effects, which are utilized for management of childhood seizures.1,2 These neurotropic effects may manifest during the initial days of KD as insomnia, restless legs syndrome, and altered mood, including hypomania (personal observation). Empathic explanation regarding the benign and self-limiting nature of these symptoms is helpful.
Long-term complications include growth retardation in children, hyperuricemia, kidney stones, and osteoporosis.21 Multivitamins, potassium citrate supplements, calcium/vitamin D supplements, and adequate hydration help in preventing these side effects. Rapid weight loss may be associated with formation of gallstones: this is possible with KD as well.
Duration of ketogenic diet
There is no consensus regarding the optimal duration of KD for management of obesity or diabetes.22 This decision must be individualized, based upon therapeutic goals, health status, and ability/willingness of the patient to conform to the suggested therapeutic diet. Well-conducted studies report safety over 2 years of use,23 though the diet can be continued for longer to take advantage of its metabolic benefits.
Conclusion
The review provides an overview of KD as a therapeutic approach in the management of obesity and diabetes. It discusses the definition and rationale of KD, its indications and contraindications, and the need for detailed preprescription screening and counseling. The advantages and limitations of KD are highlighted, as are its side effects. The KD is a therapeutic option that may be considered in persons with obesity and diabetes, under supervision of a qualified and experienced team of nutrition and endocrine professionals.