Lifestyle
Obesity has a negative impact on the reproductive outcome of women with polycystic ovary syndrome (PCOS). It affects natural conception reducing the chance of spontaneous ovulation, and increases the rate of miscarriage as well as the risk for complications during pregnancy and adverse perinatal outcomes.1–4 Lifestyle modification programs based on a combination of hypocaloric diet, increased physical activity, and behavioral interventions have shown a significant improvement in metabolic and anthropometric features (body weight, waist–hip ratio, and body composition) in women with PCOS.5 Furthermore, the reduction of excessive levels of insulin and androgens in blood leads to the restoration of menstrual regularity and ovulation, improving the reproductive function in PCOS infertile women.6
Diet with regular exercise should be recommended as first-line therapy for weight loss in overweight or obese women with PCOS (body mass index [BMI] >25 kg/m2).7 However, it seems to be beneficial for all women with PCOS, regardless of their weight and even when no weight loss occurs, as it may improve metabolic risk factors associated with PCOS.8 Overweight women with PCOS should lose at least 5–10% of their initial weight to experience significant health benefits, including spontaneous ovulation.9 Lifestyle interventions consist of a hypocaloric diet (500–1,000 calorie deficit per day) in combination with physical exercise (20–60 minutes of exercise per day from 3–5 times/week for 6 months).10,11 This is a cost-effective initial treatment strategy compared with surgical and medical options. If the patient’s compliance with lifestyle is limited, pharmacotherapy can be considered. When metformin is associated with lifestyle for a period of at least 6 months, a greater, but not statistically significant reduction, in BMI has been shown, with significantly lower subcutaneous fat and improved menstrual cyclicity compared with lifestyle + placebo.12
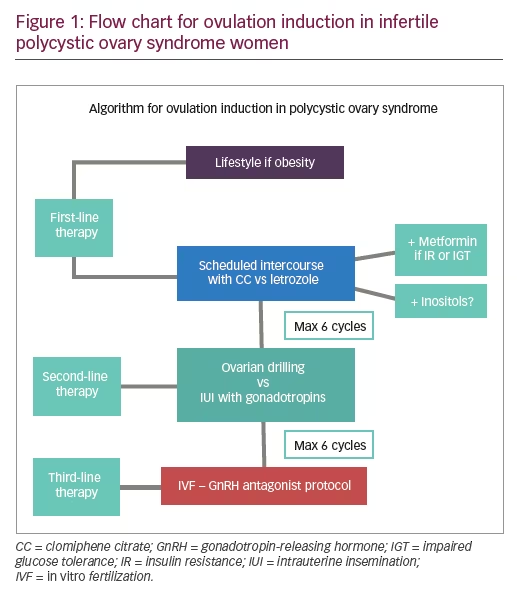
Bariatric surgery
Current guidelines suggest that bariatric surgery should be considered in obese women with a BMI ≥40 kg/m2 or ≥35 kg/m2 with associated comorbidities who have failed to lose weight with a lifestyle management program of at least 6 months.13 This surgery is not only an effective treatment in inducing and maintaining weight loss, but it could also improve fertility outcomes in obese women with PCOS, restoring regular menstrual cycles and ovulation, reducing hyperandrogenism and insulin resistance, and favoring sexual activity.14,15 Patients must be made aware of the risks of this surgery—related to the surgery itself or to the complications during pregnancy and the neonatal period. In fact, whereas the risk for gestational diabetes and large-for-gestational age infants is reduced, the risk for small-for-gestational age infants, shorter gestation, and stillbirth or neonatal death is increased.16 For these reasons, a pregnancy achieved after bariatric surgery should be considered high risk, requiring careful monitoring of fetal growth. Furthermore, patients should wait at least 12–18 months after surgery before becoming pregnant.15 This will reduce fetal complications and to overpass this initial period of rapid weight loss with nutritional deficiencies.17 Therefore, bariatric surgery should be considered the last therapeutic option in PCOS obese women with infertility. The greater the aggressiveness of the surgery, the greater the effect in reducing weight, but the greater the risk there will be for the patient as well as for her future pregnancy.
Metformin
Metformin, a synthetically derived biguanide, is the first-line oral hypoglycemic treatment for type 2 diabetes mellitus. It reduces hepatic glucose production, intestinal glucose uptake, and increases peripheral glucose uptake by skeletal muscle and liver. As an insulin-sensitizing agent it is used in patients with PCOS to reduce serum insulin concentrations and therefore improve the metabolic features of this syndrome and treat PCOS-related anovulation. The starting dose is 500 mg per day with a gradual increase up to 850–1,000 mg twice daily to manage its common gastrointestinal side effects.
Although lifestyle modification is the first-line management in PCOS obese women with infertility, metformin may be useful in weight reduction. A recent systematic review and meta-analysis including 12 studies with 608 participants has shown that the combination of metformin with lifestyle is more effective in weight loss than lifestyle alone in patients with PCOS.12 Six months of lifestyle + metformin (1.5–2 g daily) are associated with a lower BMI compared with lifestyle + placebo. When considering metformin alone versus placebo or no treatment, no effect has been shown on BMI.18 Lifestyle + metformin combined also improve menstrual cycle frequency compared with lifestyle + placebo.12 The latest update of the Cochrane review, which includes 42 trials investigating the benefit of metformin in women with PCOS, has shown higher ovulation and clinical pregnancy rates in the metformin arm compared with placebo.19
However, there is a lack of good quality evidence to support a benefit of metformin on the live birth rate. This review update includes a well-designed Finnish multicenter, double-blind, placebo-controlled trial of 320 women, who randomly received metformin, 1.5 g/day (non-obese women) or 2 g/day (obese women), or placebo for 3 months.20 If pregnancy occurred after the third month with or without another infertility treatment combined, metformin/placebo was continued up to 12 weeks of gestation. This study showed an increase in pregnancy rate from 40.4–53.6% (p≤0.006) and in live birth rate from 28.8–41.9% (p≤0.014) in women taking metformin, with the most beneficial effects observed in obese women. Therefore, 3 months pretreatment with metformin followed by the addition of another ovulation-inducing drug may increase the live birth rate in infertile women with PCOS.
Although metformin alone compared with placebo increases the ovulation rate in women with PCOS, it should not be used as first-line therapy for anovulation.21 This is because oral ovulation induction agents, such as clomiphene citrate (CC) or letrozole alone, are more effective in the achievement of ovulation, clinical pregnancy, and live birth in women with PCOS.18,22
Metformin has also been evaluated in combination with CC. The co-treatment with CC may be beneficial for women who are resistant to CC. A Dutch meta-analysis has demonstrated that the combined administration of CC + metformin significantly increases the clinical pregnancy rate (relative risk [RR] 5.6; 95% confidence interval [CI] 2.3–13) and live birth rate (RR 6.4; 95% CI 1.2–34) compared with CC alone in CC-resistant women.23 However, the latest update of the Cochrane review provides only low-quality evidence to support the increase in the live birth rate in patients receiving CC and metformin combined.19
A recent meta-analysis included 10 randomized controlled trials (RCTs) with a total of 845 infertile women with PCOS undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) treatment.24 The authors found that metformin has no clinical effect on the rate of pregnancy or live birth, but reduces the risk for ovarian hyperstimulation syndrome (OHSS). A later meta-analysis, including 12 RCTs and 1,516 patients, has shown the same results: metformin does not improve assisted reproductive technology outcomes among infertile patients with PCOS.25 The only benefit deriving from the use of metformin was the decrease in the risk for OHSS.
In conclusion, metformin represents the main insulin sensitizing agent in the management of infertile women with PCOS, with no clear benefit in improving live birth rates. It is a low-cost therapy that does not require monitoring and does not induce multiple pregnancies.26 Furthermore, it has a safety profile: no evidence of teratogenity has been demonstrated when used in the first trimester of pregnancy and no short-term adverse effects have been shown on pregnancy.27–29 Only limited information exists about long-term effects in children exposed to metformin in utero.30 Even though metformin appears to be safe during pregnancy, there is no indication to continue its use until delivery in women with PCOS as it does not reduce pregnancy complications.31 Gastrointestinal symptoms, such as diarrhea, vomiting, nausea, and abdominal disconfort, are frequent in patients receiving metformin and are a common cause of treatment discontinuation.20,32
Clomiphene citrate
CC is a selective estrogen receptor modulator that competes for receptor-binding sites with endogenous estrogens. Its anti-estrogenic effect leads to an alteration in cervical function and endometrial receptivity that might impair implantation after successful induction of ovulation. It is generally considered the first-line treatment for anovulatory women with PCOS. The starting dose is 50 mg/day for 5 days (day 3–7 of the cycle), and it may be increased by 50 mg a day up to a maximum of 150 mg/day. However, clomiphene resistance (failure to achieve ovulation after a dose of 150 mg CC per day for 5 days) is a relatively common event, occurring in approximately 15–40% of women with PCOS.33 The ovulation rate in women with PCOS after CC administration is approximately 75–80%, whereas the pregnancy rate is 22% by ovulatory cycle.34 This discrepancy of around 40% between the ovulation rate and the pregnancy rate was mainly related to the hypoestrogenic effect of CC on the endometrium and cervical mucus. CC treatment should be limited to six ovulatory cycles, as the cumulative pregnancy rate among anovulatory women with PCOS is about 65% after six CC cycles.35
In a large metanalysis,36 including 57 trials and 8,082 women, the authors have compared all of the most common regimens of ovulation induction with each other. All pharmacological treatments (CC, metformin, CC + metformin, letrozole, tamoxifen, ovarian drilling, follicle stimulating hormone [FSH]) were superior to placebo or no intervention in terms of pregnancy and ovulation rates, so expectant management is not recommended in anovulatory patients with PCOS. Compared with letrozole, clomiphene alone showed lower ovulation, pregnancy, and live birth rates. A recent systematic review37 analyzing the impact of CC and other ovulation-induction drugs on endometrial thickness in women with ovulatory disorders confirmed these results. Maybe due to the lower endometrial thickness associated, CC resulted in lower pregnancy and live birth rates than letrozole for comparable ovulation rates.
Lower fertility outcomes have also been obtained using CC compared with gonadotropins. The latest Cochrane review about this topic38 has shown that CC may reduce the chance of live birth or ongoing pregnancy in terms of gonadotropins. Although the live birth rate is higher, gonadotropins are considered a second-line intervention in anovulatory women with PCOS due to the greater probability of multiple pregnancy, the increased risk for OHSS, and their higher cost. CC is still the drug most frequently used worldwide for ovulation induction in patients with PCOS.34,39 It is administered orally, is cheap, effective, safe on offspring, with few side effects.40 Among its drawbacks, a long half-life (2 weeks) that results in long-lasting adverse effects on cervical mucus and endometrial development, and the need for careful monitoring to minimize the risk for multiple pregnancy.26
If the patient does not get pregnant after the use of CC, gonadotropins for timed intercourse or ovarian drilling are the next steps to manage anovulatory infertile women with PCOS.
Letrozole
Letrozole is the most commonly used aromatase inhibitors (AIs) for ovulation induction, representing an alternative to CC treatment, especially in those women resistant to CC. The mechanism of action consists of blocking the conversion of androgens to estrogens in the ovarian follicles, peripheral tissues, and the brain. As there is no estrogen receptor antagonism, antiestrogenic effects on the endometrium and cervical mucus are not expected, and normal negative feedback occurs generally leading to a single dominant follicle growth and monoovulation. The starting dose for letrozole is 2.5 mg a day for 5 days from day 3–7 of the cycle. The dose is increased by 2.5 mg a day up to a maximum daily dose of 7.5 mg for 5 days.
The superiority of letrozole to CC in terms of ovulation, pregnancy, and live birth rate has been shown in several clinical trials.36,41,42 A large double-blind multicenter study, including 750 infertile women with PCOS randomly assigned to receive either letrozole (up to 7.5 mg/day for 5 days) or CC (up to 150 mg/day for 5 days) for up to five menstrual cycles, has demonstrated the greater efficacy of letrozole versus CC for ovulation induction.43 Women who received letrozole had a significantly higher ovulation rate (61.7 versus 48.4%; p < 0.001) and a higher cumulative live birth rate (27.5 versus 19.1%; p = 0.007) compared with women who received CC for all BMIs, with no differences among miscarriage or twinning rates. In the subgroup of women with BMI <30 kg/m2, both treatments were equally effective in terms of live birth rate. Similarly, a recent Cochrane review44 including nine RCTs and 1,783 participants has shown increased clinical pregnancy and live birth rates using letrozole versus CC. However, no recommendation was given by the authors because the quality of the evidence was considered low. These better fertility outcomes could in part be explained through the fewer adverse effects of letrozole on oestrogen target tissues, such as endometrium and cervix, and due to the letrozole short half-life elimination time of 48 hours compared with CC.45
In conclusion, letrozole has been suggested as a possible better option than clomiphene for ovulation induction, especially in obese women with PCOS, although it is still an off-label drug in many countries.
Ovarian drilling
Laparoscopic ovarian drilling (LOD) can be used as a second-line treatment in CC-resistant women with PCOS.34 The physiopathological theory explaining the mechanism underlying ovulation induction in patients undergoing ovarian drilling is that the partial destruction of the ovarian cortex causes a local and systemic reduction in androgens, a fall in luteinizing hormone (LH) and an increase in FSH levels. These endocrine changes lead to the promotion of follicular recruitment, maturation, and subsequent ovulation.46 Ovarian drilling is commonly performed using either heat (monopolar or bipolar electrocautery) or laser with comparable outcomes.47 However, the most used technique is to perform three to eight perforations on the surface of each ovary using monopolar energy leading to further normal ovulation in 74% of the cases in the next 3–6 months.48 Several changes to the original technique have been proposed over the years.
A recent meta-analysis,49 which included eight studies with 484 participants, compared the effectiveness of unilateral versus bilateral ovarian drilling in improving fertility outcomes in infertile women with PCOS resistant to CC. No significant difference was shown not only in terms of ovulation, pregnancy, live birth, or miscarriage rates between these two techniques, but also not even in serum anti-Müllerian hormone (AMH) concentration 6 months after surgery. Therefore, ovarian drilling cortical damage does not seem to have a negative impact on ovarian reserve, although this result should be interpreted with caution due to the heterogeneity of the included studies.
The main benefit of this minimally invasive surgery compared with medical therapy is that it reduces the risk for multiple pregnancy or OHSS without decreasing the rate of pregnancy. In the last Cochrane review about this topic50 there was no difference in live birth rate when LOD was compared with gonadotropins or with AIs. Several factors could influence the efficacy of ovarian drilling. A recent review has shown that obesity, long duration of infertility >3 years, low basal LH levels <10 IU/l, testosterone level >4.5 nmol/L, and high basal AMH >7.7 ng/mL are surgery success predictors associated with poor response to LOD.51
Considering the comparable efficacy of LOD to other induction agents, this type of surgery may be an attractive option for infertile women with PCOS resistant to CC or letrozole, especially if laparoscopy is indicated for other reasons such as tubal patency assessment. The risks of LOD are all those related to a surgical abdominal procedure, including the formation of postoperative periovarian adhesions52 and a hypothetical reduction in ovarian reserve secondary to excessive ovarian damage.53 This continues to represent a controversial topic as most of the changes in the ovarian reserve markers observed after LOD could be interpreted as a normalisation of ovarian function rather than a reduction of ovarian reserve.
In conclusion, before referring a patient for ovarian drilling surgery, it is always necessary to evaluate the prognostic factors and carry out a risk–benefit cost balance.
Gonadotropins
Gonadotropins (FSH or human menopausal gonadotropin [HMG]) represent the second-line of medical treatment in women with PCOS who did not ovulate or conceive with CC and letrozole. Older anovulatory patients with PCOS could benefit from the use of a first-line treatment with gonadotropins.54
The mechanism of action of exogenous gonadotropins is to increase FSH levels in women with PCOS and stimulate follicular growth. The recommended starting dose employed to avoid hyperresponse is between 37.5–75 IU/day.34 A low-dose step-up protocol with small increases every 7 days according to follicular growth is indicated in case of no response after 14 days of ovarian stimulation. The identification of the optimal dose to be administered may require more than one ovulation induction cycle. Dosage is reduced if excessive follicular growth and/or estradiol (E2) not in accordance with follicular growth are observed. A single injection of 250 µg human chorionic gonadotropin (hCG)-r of 5,000 IU hCG-u is usually used to trigger ovulation when at least one or two follicles of at least 18 mm in their maximum diameter have developed. In the case of multifollicular growth (three or more follicles >14 mm in diameter) hCG should not be administered to prevent the risks for multiple pregnancy and OHSS. The treatment should be repeated up to a maximum of six cycles. With the low-dose step-up regimen the monofollicular ovulation rate is nearly 70%, whereas the pregnancy rate is 20% per cycle.34 Low-dose step-up and low-dose step-down protocols are more effective than the step-down conventional protocol, and both have the same pregnancy rate per started cycle.55
In anovulatory women hyperresponding to FSH during ovulation induction, the addition of recombinant LH (rLH) to FSH in the late follicular phase may lead to the dominance of a single follicle, inducing the atresia of secondary follicles.56 In fact, LH activity in menotrophin preparations for ovulation induction seems to modify follicular development, leading to a significant reduced number of intermediate-sized follicles.57
A recent Cochrane review, comparing the effectiveness of different gonadotropin preparations for ovulation induction in women with PCOS resistant to CC, has shown no difference in live birth rates and OHSS incidence using urinary FSH, recombinant FSH, or HMG/highly purified-HMG, although the quality of evidence was low or very low.58 Thus treatment outcomes depend more on the administered dose of gonadotrophins than on the preparation used.59
The combined therapy with metformin has shown good fertility results. Metformin co-treatment may improve the effectiveness of ovulation induction with gonadotrophins, increasing live birth rates compared with gonadotropins alone (odds ratio [OR] 2.46, 95% CI 1.36–4.46).60
Although time to pregnancy is shorter and pregnancy and live birth rates are higher after ovulation induction with low-dose FSH than with CC administration, gonadotropins are more expensive and have more adverse effects (OHSS, multiple pregnancies) than CC, so we always should balance the pros and cons of using one drug over another. In addition to the high cost, the other main drawback is the need for laboratory and ultrasound monitoring performed by a specialist.
In vitro fertilization
Ovarian stimulation and IVF are considered the third-line treatment for infertile women with PCOS. IVF is especially recommended if there are additional infertility factors, such as tubal damage, advanced woman age, severe endometriosis, and male subfertility.34 A single embryo-transfer procedure markedly reduces the risk for multiple pregnancy, which is one of the main drawbacks of using gonadotropins.
Despite a higher cycle cancellation rate in patients with PCOS, clinical pregnancy and the live birth rates are comparable to those of women without PCOS.61,62 The use of gonadotropin-releasing hormone (GnRH) antagonist limits the occurence of OHSS, whose incidence is significantly higher in women with PCOS (15%) compared with those with normal ovaries (3%).63 Women treated with the GnRH antagonist “short protocol” have a lower incidence of OHSS compared with those receiving the “long GnRH agonist regimen” (OR 0.61, 95% C 0.51–0.72; 36 RCTs, n = 7,944) without reducing the likelihood of achieving live birth (OR 1.02, 95% CI 0.85–1.23; 12 RCTs, n = 2,303).64
Another important advantage of the GnRH antagonist protocol is that it allows the final oocyte maturation with a bolus of GnRH agonist to be triggered instead of hCG, a procedure that significantly reduces or totally eliminates the risk for OHSS in high-risk patients. GnRH-agonist administration for final oocyte maturation is associated with a luteal phase defect that leads to a significantly reduced likelihood of ongoing pregnancy achievement compared with standard hCG treatment.65 No difference regarding the number of oocytes retrieved, the proportion of metaphase II oocytes, the fertilization rate, and embryo scores were found when hCG and GnRH agonist were compared.66 After GnRH agonist triggering, the best option is to freeze all embryos and transfer them in a subsequent frozen-thawed embryo replacement or natural cycle.67 Nowadays, the combined use of a GnRH antagonist protocol with GnRH agonist triggering and embryo freezing should be recommended as a standard preventive measure of OHSS in patients with risk for ovarian stimulation hyperresponse.
Inositol
Inositol is a sugar alcohol with multiple stereoisomers. Inositols are involved in the postreceptorial signal transmission of different hormones such as insulin, TSH, and FSH, and their metabolites have a relevant physiological role in reducing insulin resistance and hyperandrogenism, and have beneficial effects on reproductive function,68–72 so inositol supplementation has been proposed to improve reproductive outcomes in infertile women with PCOS.
The two most commonly studied isomers, myoinositol (MI) and d-chiroinositol (DCI), control key enzymes involved in glucose and lipid metabolism. DCI is mainly involved in glucose uptake and glycogen synthesis (liver, fat, and muscle), while MI is responsible for the activation of gluco-transporters and glucose utilization. MI is more beneficial in improving the metabolic profile; DCI is more effective in reducing androgen levels and modifying endocrine parameters.73 Treatment with MI is effective not only in improving insulin resistance but it has also shown a significant advantage in terms of reduction of BMI.74,75 The probability of ovulation and the chance of pregnancy are reduced in obese women with PCOS treated with MI compared with normal–overweight patients, and no metabolic benefits of MI treatment have been observed in obese patients with a BMI >37.75,76 However, an Italian study has shown that MI administration is more effective in those obese patients who have high fasting insulin plasma levels,77 whereas DCI administration has better results in those obese hyperinsulinemic patients with PCOS who have a history of relatives with diabetes.78
A recently published systematic review and metaanalysis of 10 RCTs including 601 women with PCOS79 has shown that inositol supplementation increases ovulation rate and menstrual cycle frequency compared with placebo. Whether used alone or in combination with CC in MI-resistant patients, MI produces very good clinical results, with an ovulation rate of 61.7% and 72.2%, respectively, when administered during three spontaneous menstrual cycles.75 However, these results do not seem to translate into better reproductive outcomes. In the last review about inositol supplementation in women with PCOS undergoing ICSI, the authors concluded that MI does not improve oocyte or embryo quality and pregnancy rates, and that DCI has a controversial role.80 When administered, a recent international consensus conference recommends using a combination of both inositol isoforms in their physiological plasma ratio 40:1 to have benefits at systemic and ovarian levels.81
Recently, alpha-lipoic acid treatment, alone or in combination with MI or DCI, has shown to improve the metabolic parameters of obese patients with PCOS modulating glucose utilization through the activation of adenosine monophosphateactivated protein kinase in skeletal muscles thus increasing glucose-transporter-4.82 Data from Genazzani’s group demonstrated a significant decrease in insulin, glucose, BMI, and Homeostasis Model Assessment index parameters after 12 weeks of daily intake of 400 mg of alpha-lipoic acid.83
Conclusion
Different treatment options exist to achieve ovulation in women with PCOS. The clinical decision of which treatment recommend should be made on local facilities, adverse effects, cost, and patient compliance. Ovulation induction has to be individualized according to the patient weight and PCOS phenotype, with the aim of achieving mono-ovulation and subsequently the birth of a singleton healthy baby, minimizing the risks for OHSS, and multiple pregnancy.