Introduction
Diabetes mellitus (DM) is a group of metabolic disorders characterized by high blood glucose levels. According to International Diabetes Federation (IDF) 2017 data, there were 425 million persons with DM worldwide, and that is expected to reach 629 million by 2045.1
Normal sexual health is a dynamic and harmonious state involving erotic and reproductive experiences, and fulfillment within a broken physical, emotional, interpersonal, social, and spiritual sense of well-being, in a culturally informed, freely and responsibly chosen and ethical framework; not merely the absence of sexual disorders.2 Female sexual dysfunction (FSD) is a complex set of conditions associated with multiple biological, medical, psychological, and social risk factors.3,4 The World Health Organization (WHO) defines FSD as “the various ways in which a woman is unable to participate in a sexual relationship as she would wish.”5 Desire, arousal and orgasm are three principle stages of sexual response cycle.6 FSD is characterized by lack or loss of desire, sexual aversion, lack of sexual enjoyment, vaginal dryness, markedly delayed or nonexistent orgasm, vaginismus, and dyspareunia. A clinically useful definition of FSD is “the persistent/recurring decrease in sexual desire or arousal, the difficulty/inability to achieve an orgasm, and/or the feeling of pain during sexual intercourse.”3,6
DM is an important cause of sexual dysfunction (SD) both in men and women.7 SD is more difficult to diagnose and treat in women than it is in men because of the intricacy of the female sexual response.8,9 Although SD in males with DM is widely explored, there is limited literature regarding FSD and DM and it is often a neglected health issue in women with diabetes.7 However, it affects the overall quality of life, physical and emotional health, and deserves more attention in clinical practice as well as in clinical research.10 The aim of this review is to update the scenario of prevailing SD faced by the women with DM, their etiology, diagnostic approaches, and its management.
Prevalence of female sexual dysfunction
SD is common in people with DM.11 The global prevalence of SD in women with DM is estimated to be 20–80%.12,13 A systematic review published in 2016 by McCool et al. estimated the global prevalence of FSD among premenopausal women to be 40.9% (95% CI 37.1–44.7, I2 = 99.0%).14 SD also increases in transition from early to late menopause.15 Systematic reviews and meta-analyses have reported a higher frequency of FSD in women with DM.16,17 A meta-analysis by Pontiroli et al showed a higher frequency of SD among women with type 1 DM (T1DM; OR [95%CI] 2.27 [1.23, 4.16]), T2DM (OR [95% CI] 2.49 [1.55, 3.99]), and in any diabetes (T1 and T2; OR [95% CI] 2.02 [1.49, 2.72]) than in control women.16
However, data from conservative societies like Middle Eastern and South Asian countries is limited. A cross-sectional study conducted in Iran (2012–2013) reported that 53.6% women (age 30–55 years) with T2DM had FSD.18 In a North Indian study 45.19% women suffered from desire disorder, 62.71% from arousal disorder, 84.75% from orgasmic disorder, and 20.38% from pain disorder. Also, significant number of women had lower self-esteem, felt less attractive, accepted their detrimental sexuality and believed in their reduced “desirability” in their spouse’s eyes’. Only 3.95% of women had previously discussed these issues with a healthcare provider, while 83.59% would prefer this type of counseling with their diabetes care team.19,A study conducted in South India has shown that sexual problems seems to be more prevalent among less educated women with age above 40 years.20
Etiology
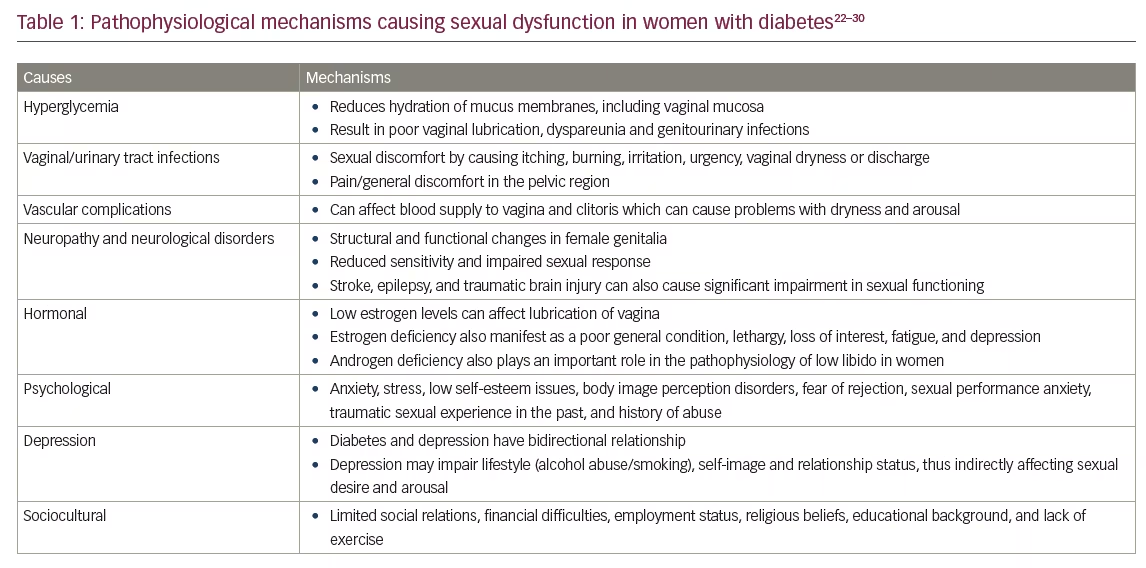
FSD is multifactorial disorder involving subjective and objective aspects of sexual function, with numerous contributing factors (hormonal, psychological, interpersonal, and social). DM is known to cause different medical, psychological, and sexual complications. Evidence shows significant association between female sexual health and vascular risk factors (hypertension, hyperlipidemia, metabolic syndrome/obesity, diabetes, and coronary heart disease).21,22 Women with DM may have less sexual desire which can be due to depression or changes in blood glucose levels that can leave them feeling tired or irritable.21,23 FSD can also be an early symptom that leads to the diagnosis of DM.11 The pathophysiological mechanisms that play an important role in the causation of FSD among persons with DM, have been summarized in Table 1.22–30
FSD in persons with DM has repeatedly shown to be correlated with psychological distress.31 Significant ethnic and gender differences are known to exist in acceptance of disease, receiving social support, disease knowledge and perceived difficulty in self-management behaviors, glycemic control, and quality of life among T2DM patients. This negative appraisal is independent of duration of diabetes.26 Improved education and support for women with DM may result in better disease management, a more positive outlook regarding their health, and thus an improvement in sex life.
Additionally, FSD is correlated with lower education, lower income, urinary tract symptoms, cancer survivors, history of sexual abuse, trauma to genitalia, use of antidepressants, and disturbed spousal support.6 Many women in conservative societies such as South Asia experience inaccessibility of trained female healthcare professionals, lack of privacy and confidence, and a social taboo attached to the subject of sexual health.31 This is compounded by health and gender inequity and a lack of resources or medical services.
Symptoms and signs of female sexual dysfunction29,32,33
- Hypoactive sexual desire: decrease or loss of desire for sex
- Inadequate vaginal lubrication before and during intercourse
- Inability to achieve orgasm
- Inability to relax the vaginal muscle enough to allow intercourse (vaginismus)
- Pain during intercourse: vulvodynia
- Non-coital sexual pain disorder (recurrent or persistent genital pain induced by non-coital sexual stimulation)
Screening and diagnostic methods
To identify whether a diabetic woman has sexual dysfunction, a detailed history and examination is the first step. This can be done by their primary care physicians or anyone from their diabetes care team.32 The consultation should focus on the woman’s current interpersonal and psychosocial status, her sexual and medical history, comorbid illnesses, enquiries about sexual abuse or other traumatic sexual experiences, number of sexual partners, impact of religious and social beliefs on sexuality, as well as her current diabetes treatment.4 History should be inclusive of menstrual abnormalities and infertility. Diabetes has been associated with menstrual abnormalities, such as oligomenorrhea, secondary amenorrhea, delayed menarche, and premature menopause which diminishes the patient’s reproductive period. Evidence shows that better glycemic control improves such irregularities and increases fertility rates.34 History and examination needs to be done in comfortable environment with use of flexible conversations and also structured interviews. Several self-reported validated questionnaires such as Female Sexual Function Index (FSFI; most frequently used in research), the Brief Index of Sexual Functioning for Women (BISF-W), the Derogatis Interview for Sexual Function (DISF/DISFSR), and the Female Sexual Distress Scale (FSDS) have been developed to assess FSD in clinical practice as well as for research purpose. The principal components of the most commonly used scale, FSFI, include desire, subjective arousal, lubrication, orgasm, satisfaction, and pain.32,35 Similarly two models have been proposed namely ALLOW (Ask, Legitimize, Limitations, Open up, Work together) and PLISSIT (Permission, Limited Information, Specific Suggestions, Intensive Therapy) consisting of simple domains to facilitate further evaluation by probing about sexual life, discussions, limitations, specific suggestions and intensive treatment.36
Selected women may need laboratory work up for hypothalamic-pituitary-ovarian axis and the hormonal status including hyperandrogenism. Several possible determinants of FSD including autonomic neuropathy, vaginitis, pelvic inflammatory diseases, urinary tract infection, and female genital mutilation, can be diagnosed by good history, examination, and selective work up.37
Treatment
Treatment of FSD depends on underlying etiology and is a multi-disciplinary task. Treatment options are summarized in Table 2.21,38,39 Intensive lifestyle interventions in terms of diet and exercise have shown beneficial effects on sexual functioning especially among obese women with diabetes.40 A healthy diet, routine exercise, avoiding smoking, and usage of appropriate therapies for optimum glycemic and metabolic control (including control of hypertension and dyslipidemia) are the key to preventing and managing progression of diabetes-induced sexual problems.23 Omega-3 fatty acids may be beneficial in general well-being, improvement in libido, and prevention of menstrual syndromes, such as dysmenorrhea and postmenopausal hot flushes by reducing the production of eicosanoid pro-inflammatory molecules.41,42 Many cases of FSD can be treated with simple interventions like treatment of and underlying genital infection. Vaginal lubricants and moisturizers are helpful for vaginal dryness in women with DM who are postmenopausal, or those with hyperglycemia who complain of vaginal dryness or dyspareunia.
Limited evidence has shown that dehydroepiandrosterone (DHEA) may improve libido and well-being among postmenopausal women. It provides a large precursor reservoir for the intracellular production of androgens and estrogens in non-reproductive tissues, restoring the circulating levels of these steroids to improve well-being and sexual function.43 Flibanserin, a serotonin 1A receptor agonist and a serotonin 2A receptor antagonist, is the only US Food and Drug Administration (FDA)-approved agent for generalized acquired hypoactive sexual desire in premenopausal women. However, patients need to be counseled for increased risk of severe hypotension and syncope. Also, data in women specifically with DM is limited.44,45
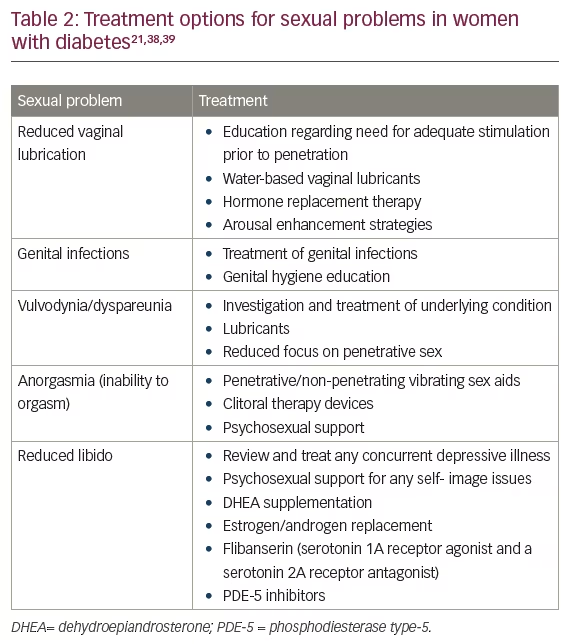
Certain medications may enhance the vasocongestive response for women with genital arousal disorder. Systemic as well as transdermal estrogen replacement and androgen replacement are being used to increase sex hormone binding globulin, reduce bioavailable testosterone, improve genital response, and improve the mental response.44–46 Further, phosphodiesterase inhibitors are upcoming treatments to improve sexual function but require future investigations for safety and effective concerns.47,48
Future directions
Further studies are needed to better understand occurrence of SD and its impact on quality of life among women with DM, especially from conservative societies. Future studies may identify appropriate medical professionals to probe and explore the related issues for early screening. More studies are needed before the role of newer treatments, such as DHEA supplementation, estrogen/androgen replacement, and flibanserin can be established.







