World Health Organization statistics rank tuberculosis (TB) amongst the top 10 communicable diseases in the world, and it remains one of the biggest killers of mankind.1 TB is known to cause both mortality and morbidity. Pulmonary TB is the primary manifestation, but genital TB is also found in a considerable number of men and women. There have been reports detailing an increase in cases presenting to gynecological clinics, partly due to a growing population and partly due to an overall rise in patients with TB.2 Female genital TB is said to be concomitantly present in 10% of all pulmonary TB cases, and accounts for 5% of all female pelvic infections.3 Similarly, about 10% of men may present with TB and infertility.4
Genital TB remains a diagnostic dilemma, primarily because of its insidious nature and virtually symptomless presentation, especially in early stages. It often goes undetected, even though genitourinary TB is acknowledged as the third most common site for infection, after the lungs and lymphadenopathy.5 Infertility, at times, is the only presenting symptom of this disease, both in men and women, and it is usually incidentally uncovered during investigation for infertility.6,7 Males may present with culture sterile semen with pus cells, while women commonly may have menstrual irregularities. It is therefore important to recognize both the active and the latent, or dormant, state of the disease.
The aim of this review is to collate available data on genital TB in both sexes, in order to understand its impact on fertility and how the disease makes the individual functionally incompetent to reproduce.
A literature search was performed using PubMed (1966–2020) using the following search terms: “female genital tuberculosis”, “male genital tuberculosis”, “genital tuberculosis”, “infertility”, and “latent genital tuberculosis”. A total of 6,081 articles were retrieved. Of these, 427 relevant articles were screened. Further searches were made for diagnosis and management. Cross-references were also searched for appropriate general terms, like “latent tuberculosis”.
Incidence
Due to its subtle presentation, the incidence of genital TB is not known. It is, however, estimated that 5% of females presenting themselves to infertility clinics worldwide have genital TB, and the majority (80–90%) of these are aged 20–40 years; however, older women are also known to harbor it.8 On the other hand, 4.0–9.1% of men with infertility with a clinical diagnosis of obstructive azoospermia have their diagnosis attributed to male genital TB as the cause.9,10
Female genital tuberculosis
Chronic infections, like TB, may cause both anatomical and physiological damage to the female reproductive system, thereby affecting reproduction as well as the production of hormones. While the process of reproduction is limited to the performance of the genital tract, a dysfunction in the production of hormones may have far-reaching consequences all across the body. This can happen as a consequence of the active disease, but may also continue in its latent or dormant state. Premature ovarian failure is now a well-recognized consequence of female genital TB, and in addition to infertility, a deficient estrogen state can result in premature menopause with its accompanying problems.11
Active female genital tuberculosis
Active TB in the genital tract may present singularly or as a part of abdominal TB with low-grade fever, loss of appetite and weight, and pain in the lower abdomen not conforming to any specific pattern. There may be tenderness in the lower abdomen and a doughy feel to the abdomen. Ascites may also be present. A local examination may reveal vaginal discharge12 and a cervical ulcer,13 which may be diagnosed on Pap smear or an acid-fast stain for acid fast bacillus. The uterus may be fixed and tender to touch with fullness or masses in the fornixes. The disease can be diagnosed by a smear, endometrial biopsy, or histopathology and culture.
Often, the disease goes undiagnosed or misdiagnosed, and is treated as chronic non-specific pelvic inflammation. In the chronic state, one may find vague symptoms, such as a painful abdomen and dyspareunia. Local examination may reveal tubo-ovarian masses, but investigations such as ultrasound scans, hysterosalpingogram, and laparoscopy and hysteroscopy may show pathognomonic findings.14 Tissue sampling can help to detect the bacillus via new molecular tests or histopathological examination, which may reveal findings specific to TB.
Latent, or dormant, female genital tuberculosis
Latent TB infection is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifesting active TB. There is no gold-standard test for latent TB infection.15 The concept of TB remaining in the body as a latent infection was first understood when it was reported that TB is widespread in patients with human immunodeficiency virus (HIV).16 The disease was found to surface when the immunity of the person was low. The heightened immune system of a mother infected with TB not only controls the infection, but at the same time does not allow the acceptance of the foreign proteins of sperm or embryo as its own, thus leading to infertility.17
In 1992, Chandrasekhar and Ratnam confirmed that inert forms of Mycobacterium tuberculosis replicate into active forms of the bacteria under favorable conditions.18 Later, Chan and Flynn elaborated upon the etiopathology of the various stages of the infection, and how it evades the host’s immune system and survives in the body.19 In situations where the host response breaks down, Mycobacterium tuberculosis is activated, thus progressing from a dormant stage to an active form.
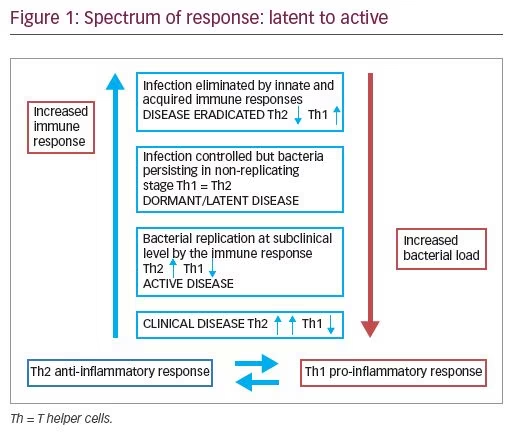
Latent TB, therefore, comprises of a diverse range of states, or a spectrum: from those who have completely cleared the infection to those who are incubating actively replicating bacteria in the absence of clinical symptoms. Active TB, on the other hand, exhibits multiple presentations at the pathological level, ranging from small granulomas, to caseous lesions containing variable numbers of bacteria, to liquefied cavities with a massive load of replicating organisms, to miliary disease. The presentation reflects the state of immunity of the individual.
Anatomical changes and clinical presentation
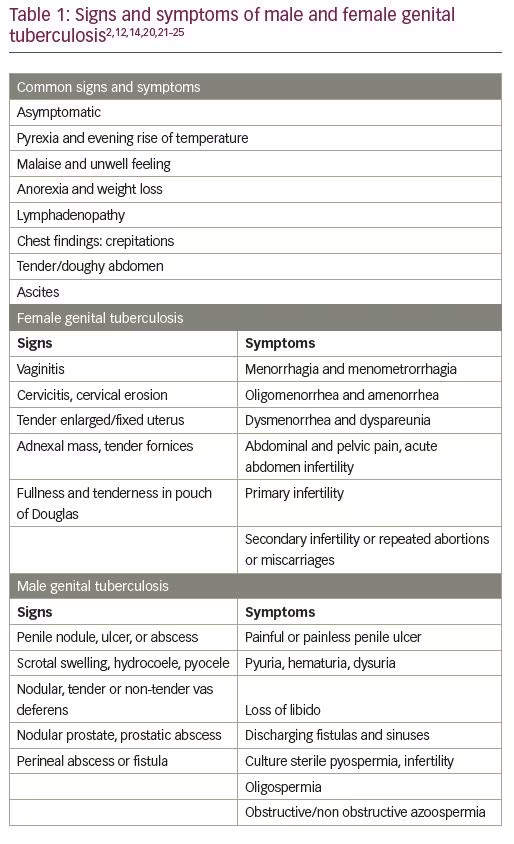
Genital TB may be found in high-risk groups, such as those with infertility; recurrent miscarriages; ectopic pregnancies; menstrual irregularities, including menorrhagia, oligomenorrhea, and amenorrhea; adnexal mass chronic pelvic pain; family history of TB; and history of previous TB.20 About 20% of patients with genital TB have a family history of TB in an immediate family member.8 Upon careful questioning, about 30–40% report previous pleural effusion, peritonitis, osseous, lymph node or pulmonary TB. Infertility associated with an adnexal mass may be caused by TB in about 39% of cases.14 The fallopian tubes are the likely site of initial infection in the majority of the cases, and bilateral involvement of the fallopian tubes is common.2,5,14 Tubercular salpingitis and hydrosalpinx are well-known and established causes of infertility.21 The uterus is said to be involved in 50–70% of cases, predominantly affecting the endometrium and occasionally the myometrium.2,5 Amenorrhea or oligomenorrhoea due to Asherman’s syndrome are also known entities. The ovaries may be involved in 20–30% of cases.11 All these states can lead to permanent disfigurement of the genital tract, and loss of fertility (Table 1).2,12,14,20,22–25
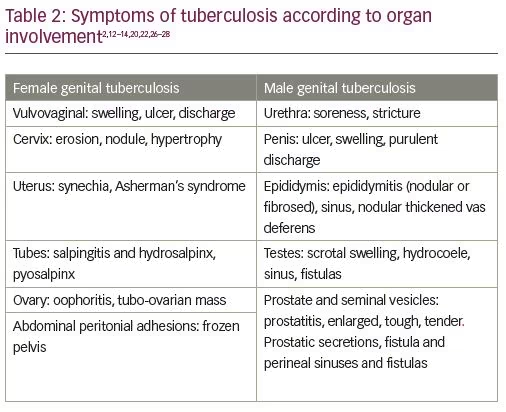
TB of the cervix is uncommon and may present as a growth or an erosion Table 2).2,12-14,20,22,26–28 However, it is pertinent to state that these lesions may not always indicate active disease. Many of these are sequelae of active disease that have been previously treated. Many cases of genital TB may not have any signs or symptoms at all, and may be detected during routine investigation of infertility. This condition has been described as latent, or dormant—as discussed above. Female genital TB can be seen to pass through various phases—latent, active, burnt-out, or complete cure (Figure 1). It is therefore important to recognize the infection in its latent state before irreparable damage is done and there is permanent sterility.
Physiological and immunological changes
An increasing interest in reproductive endocrinology and the advent of assisted reproductive technology has led to a better understanding of the role that chronic infections play in fertility. The ensuing oophoritis, as a result of TB, may deplete the ovarian reserve and lead to ovarian insufficiency.26 An anti-gonadotrophic effect has also been reported in pulmonary TB, thereby causing secondary ovarian failure in such women.29 An anti-estrogenic effect has been observed in patients taking contraceptive pills, where the efficacy of pills was decreased leading to failure of contraception.30
In a study investigating the reactivation of pulmonary TB, it was found that, as the number of mycobacteria isolated from the lung increases following activation of the hypothalamic–pituitary–adrenal (HPA) axis, there is a change in cytokine production by T cells from predominantly type 1 cytokines to a type 2 cytokine profile.31 While the cytokines produced by T cells in the lung during latent infection were predominantly type 1—i.e., interleukin (IL)-2- and interferon (IFN)-γ-producing cells—HPA activation resulted in a shift to IL-4- and IL-10-producing cells. This indicates that the disease is controlled, to an extent, by hormones, and remains contained when there is a Th1 immune reaction and flares up when there is a shift to a Th2 immune response, as happens in pregnancy (Figure 1).
Local suppression of hostile immune responses in the uterus is necessary for fetal acceptance, which is a semi allograft containing paternal antigens. It is now well known that immune regulation is brought about by complex immune–immune interactions as well as immune–endocrine interactions to ensure fetal survival within the maternal uterus. Reproductive hormones, such as progesterone, estradiol, and human chorionic gonadotropin, regulate the menstrual cycle and establish and maintain pregnancy, and also terminate it at the opportune moment.32 These hormones inhibit destructive immune responses and induce tolerance by binding to their specific receptors expressed by immune cells and/or by acting via mediators. This includes the reduction of the antigen-presenting capacity of dendritic cells, monocytes, and macrophages, as well as the blockage of natural killer cells, T cells, and B cells.33 Pregnancy hormones also support the proliferation of pregnancy by supporting uterine killer cells, retaining tolerogenic dendritic cells, and efficiently inducing regulatory T cells. The endocrine factors, therefore, play a major role in immune tolerance in pregnancy. Depending upon the levels of the two major hormones estrogen and progesterone, there is upregulation and downregulation of a battery of cytokines.32 The aim of this immunomodulation is dual—to accept the semi allograft, the embryo, and simultaneously safeguard the mother against offending organisms, such as Mycobacterium tuberculosis.33 Any perturbations in hormones would therefore result in infertility.
Activation of latent female genital tuberculosis to disease
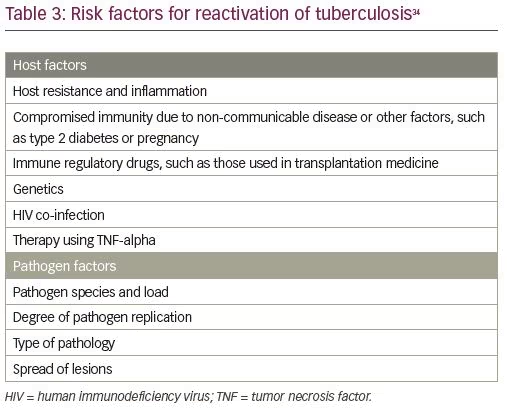
As described above, females with latent TB infection are at an increased risk of progression to active TB. Many factors attributed to the host and the pathogen have been described that are responsible for reactivation of the disease (Table 3).34 However, a large number may not develop TB, either because of an efficient immune system or because they are no longer infected with live bacteria. There is also increasing evidence that familial genetics of the host influence its disease manifestations, fulminant spread, and outcomes.35 An attempt to make an infertile patient pregnant may activate the latent infection.36,37 Reactivation can also be initiated during surgical manipulation, and has been observed post laparoscopy, hysteroscopy, hysterosalpingography,38,39 and pelvic surgery.40 High steroid levels and an increased vascularity during ovarian stimulation are thought to be the triggering factors in patients who are infertile and going through in vitro fertilization (IVF). Empirical use of steroids and immunotherapy is common in patients with infertility with recurrent implantation failure and recurrent pregnancy loss, and these too increase the risk for reactivation.41 Immunocompromise always remains the most important cause of reactivation of a dormant bacterium, as seen in individuals who are HIV positive.42
Male genital tuberculosis
Male genital TB is commonly found secondary to a pulmonary infection. It commonly affects young males in their reproductive years, aged 29.6–32.0 years.22,43
Active and latent male genital tuberculosis
Genital TB has not been studied as extensively in males as it has in females, primarily because of the different roles in reproduction. While females provide both the gamete and the uterus to carry the pregnancy, the male provides just the gamete. As such, the genital TB not only affects conception in females, but also the ability to carry the pregnancy. The anatomical changes that take place as a result of TB have been well defined in males, but there are very few reports on the changes in physiology and endocrinology. The latent form of the disease has not yet been recognized as an entity, even though there are reports of asymptomatic men presenting with adverse semen reports.7 Clinical findings at the time of primary infection have been well elucidated, but those of the subclinical or dormant stages are less clearly defined. However, its impact on hormones and immunity are yet to be demonstrated. Scant reports of its anti-gonadotropic effect and resultant loss of libido can be found.23,44
The subclinical pulmonary infection primarily spreads hematogenously into the kidneys, epididymis, and the prostate. The epididymis and kidneys are bilaterally involved. As immunity develops, these lesions cicatrize in about 6 months and a latent phase follows. Similarly to female genital TB, reactivation of these latent foci due a decreased host immunity may transpire. This time span has been seen to be longer in men, ranging from 1 to 46 years.27 Reactivation of the latent bacilli may either occur in the epididymis or the prostate in 22–55% of cases,27,45 or by secondary spread from the already infected genitourinary organs via the urinary system, by canalicular spread (both antegrade and retrograde), or by lymphatic spread.46 The testes are involved by direct extension from the epididymis due to the blood–testes barrier.47 Seminal vesicles are never isolated in their involvement, which is by the canalicular route. Despite the urethra’s constant exposure to infected urine, urethral TB is rare, but reports of penile TB are common.48
Penile TB has been described as a sexually transmitted disease, transmitted from a sexual partner infected with active pulmonary disease.24,48,49 Sexual transmission has been demonstrated in the semen of the patients with pulmonary as well as prostatic TB, showing identical organisms isolated from a penile ulcer and endometrial biopsy of a couple.24,48,49 Reinoculation of the male partner through his own infected ejaculate and secondary involvement via the urethra may also lead to the development of penile lesions in a patient with genital TB.24 Therefore, sexual transmission of the disease is a possibility.
Anatomical changes and clinical presentation
About 10% of patients with male genital TB may present with infertility, and around 4.0–9.1% of such men may have obstructive azoospermia.9,10 Clinical presentation can range from a painless scrotal mass in common cases, to irritative lower urinary tract symptoms, hematuria, dysuria, pyospermia, hemospermia, infertility, or ulcerative penile lesion.50,51 On the contrary, patients may have no symptoms at all except infertility, and the disease may be incidentally diagnosed on histopathology or picked up on finding a poor quality semen with sterile pyospermia or complete azoospermia. Many patients may subsequently report a past history of TB in themselves or in a close contact, or they may be immunocompromised, such as through HIV, or may just be residing in an endemic region.
Infertility resulting from genital TB in men is destructive in nature, with scarring and fibrosis that may persist even after successful medical management.9,25 The epididymis and vas generally get obstructed, either by the granulomatous masses in the acute phase or by fibrosis and scarring as the disease progresses, or post therapy.25 A bilaterally enlarged nodular epididymis and vas deferens, which is indurated and with or without sinus formation on local examination, suggests TB. In cases with isolated epididymal or vas involvement, semen parameters may show obstructive azoospermia, or reveal azoospermia or severe oligospermia with normal volume fructose-positive ejaculate.52 In such cases, the hormonal profile and spermatogenesis is normal.
On the contrary, tubercular inflammation or scarring of the prostate, seminal vesicle and ejaculatory ducts will clinically present as low volume fructose-negative ejaculate, thus mimicking ejaculatory duct obstruction. Male genital TB may also present as an unexplained, gradually progressing decline in the volume of ejaculate associated with azoospermia and progressing to aspermia.53 A transrectal ultrasonogram can aid in assessing seminal vesicles, which are fibrotic and atrophic in TB.25,54 Thus, ejaculatory duct obstruction with non-distended atrophic seminal vesicles is diagnostic of TB, and most of these patients have multifocal obstruction, for which assisted reproduction is beneficial rather than surgical intervention.10,54 However, few patients have obstruction at the level of ejaculatory ducts with dilated seminal vesicles.25 These patients can be offered surgical correction in the form of transurethral resection of ejaculatory duct (Table 2).2,12-14,20,22,26–28 Most patients with tubercular infertility require assisted reproduction, IFV, or intra-cytoplasmic sperm injection53,54 as multiple organ involvement with obstruction at several sites is characteristic of genital TB and renders the case inoperable.10,54 Testicular sperm extraction may be carried out if no sperm are obtained from the epididymis. The outcomes of sperm retrieval and pregnancy are similar between tubercular and non-tubercular causes of obstructive azoospermia.55
In cases of involvement of the testes, there may be atrophy of the testes noted with caseating granulomatous inflammation on histopathological examination.56 Such patients may not show improvement in semen parameters post anti-TB therapy, and surgical retrieval of sperm may not reveal any results, hence third party reproduction is recommended.
Diagnosis of genital tuberculosis
It is a challenge to diagnose genital TB, despite the battery of tests currently available. The guiding principle for the diagnosis of any infectious disease is documentation of the offending organism in the tissue in question. Female genital TB is a paucibacillary disease, and as such it is very difficult to find the bacteria within the tract. An acid-fast bacillus culture or presence of bacteria in the tissue or fluid is, even today, considered the gold standard. Indirect evidence, such as ultrasound, magnetic resonance imaging, laparoscopy, and hysteroscopy,57 are used as diagnostic modalities. The sensitivity most tests is poor, ranging from 5–7%, and many cases of early disease are missed.58 Mahajan et al. have reviewed the pitfalls and controversies around the tests for Mycobacterium tuberculosis in detail and concluded that a battery of tests may be required to make a diagnosis in the majority of cases.59 An algorithmic approach using multiple tests, signs, and symptoms has therefore been advocated by healthcare professionals.41,60
A promising, more specific test is an updated version of the QFT-GIT (QuantiFERON®-TB Gold In-Tube [QIAGEN, Hilden, Germany]) test that contains peptides which are intended to specifically induce a CD8+ T-cell response in addition to the CD4+ T-cell response. The rationale is that Mycobacterium tuberculosis-specific CD8+ T cells are more frequently detected in people with active TB compared to latent TB infection; they are associated with a recent exposure to TB and they decline when patients receive anti-TB treatment. The first data on performance of QFT-GIT in a multicenter European study were reported recently.34 Other biomarkers, in addition to this, have also been reported.61
Treatment
Medical management
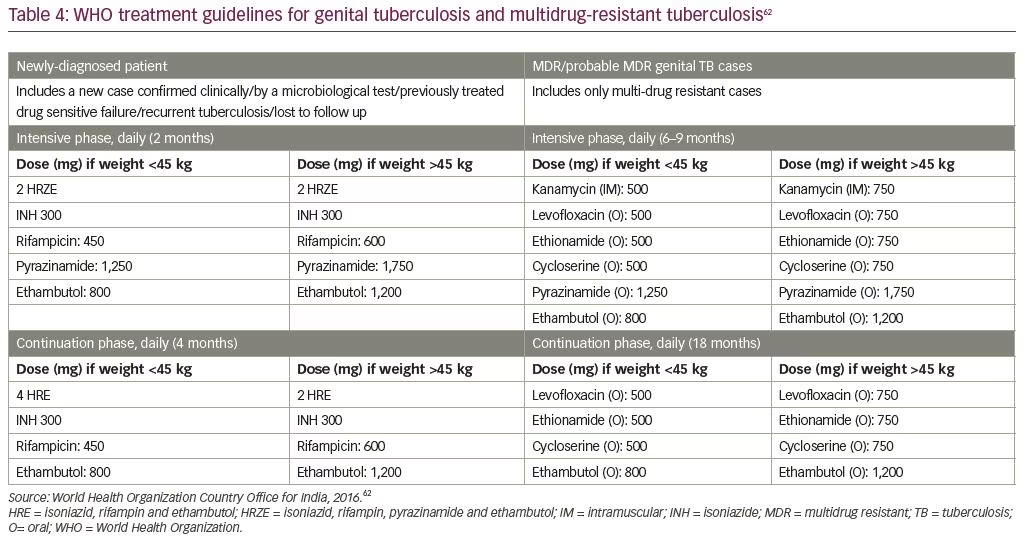
Medical management according to the ovarian reserve is the mainstay of treatment on confirmation of TB.28 Guidelines for the treatment of extrapulmonary TB were also developed and released in India in 2016.62 The standard drug regimen may be given with a four-drug regime for 2 months followed by three drugs for 4 months (Table 4).62 Cases of multidrug resistant and extensively drug-resistant TB are difficult to identify because of the paucibacillary nature of the disease. However, in the event that a TB nucleic acid amplification test is positive, drug sensitivity may be assessed and second-line therapy can be initiated.
Surgical management
Surgery is indicated in the following situations for fertility enhancement in female patients: hydrosalpinx and pyosalpinx, tubo-ovarian masses and abscesses, pelvic adhesions and peritonitis, and Asherman’s syndrome. It would also be required in some cases of ectopic pregnancy. In male patients surgery is indicated only if there is a failure of medical treatment, abscess formation, or obstruction of the vas. Orchidectomy may be required in some cases of testicular abscesses that do not resolve with medication. Some patients have obstruction at the level of ejaculatory ducts with dilated seminal vesicles, for which surgical intervention may be required.25
Assisted reproduction
In females, assisted reproduction is indicated in patients with blocked fallopian tubes, Asherman’s syndrome, or diminished ovarian reserve. In male patients, assisted reproduction is indicated by oligoasthenoteratozoospermia, chronic prostatitis and epididymitis, or obstructive azoospermia. Ejaculatory duct obstruction with atrophic seminal vesicles is diagnostic of TB; most of these patients have multifocal obstruction preventing surgical reconstruction, and assisted reproduction may be their only chance of conception.10,55
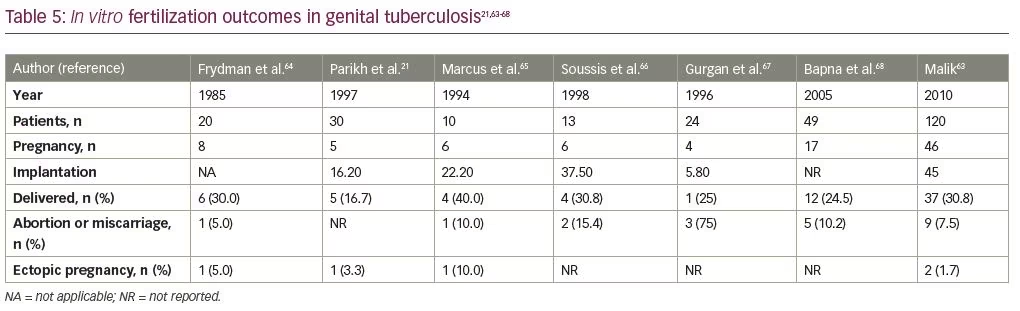
Pregnancy rates in IVF seem to depend upon the severity of the disease, and are better if the disease is diagnosed early (Table 5).21,62–67 Reports indicate poor results due to poor oocyte quality, poor ovarian response, and a hostile uterine environment.64–68 Many patients may therefore have to resort to third-party reproduction in the form of oocyte donation or surrogacy.69
Conclusion
TB is known as the “great masquerader”. This applies more to genital TB than any other form of extrapulmonary TB. While the disease itself is a dilemma, there is also a time limit for treating infertility, especially in female patients. The age-related decline in ovarian reserve and patients’ desire to get pregnant quickly is a major challenge.
This narrative review has highlighted many lapses in the understanding of this disease. There are no systematic reviews or randomized trials in this field which address both the diagnosis and management of genital TB. It is important to understand that every organ and body tissue has different functions, and any anatomical distortion leads to a change in the physiological function (including endocrinology and immunology). With advances in reproductive medicine, the impact of chronic infections on the process of reproduction is gradually being realized. Few case reports have been published on genital TB, and most of our understanding has been extrapolated from studies conducted on pulmonary TB, but this leaves many gaps in our knowledge. The need of the hour is a test that can quantify bacterial numbers or levels of replication during latent TB infection, and allow us to differentiate between latent and active disease, or even the progression from latent to active disease. There is ongoing research into identifying and testing biomarkers that may tell us which level of the spectrum the bacteria is occupying.34 This would help in the identification of those who need treatment, those in who treatment will not help, and probably even those where treatment is not required.
Genital TB is an under-diagnosed presentation of TB and has a profound effect on reproduction both in male and female patients. It is a complex chronic infection presenting as latent or active infection. The organism is known to survive inside the human body for years and can covert to its active form whenever host resistance is lowered. In its active state it can cause variable anatomic damage to the reproductive tract depending upon the virulence of the organism and host resistance, which can render the infected person infertile. The physiological impact and altered immune response that accompany it are slowly being recognized.
Early detection of the disease can prevent irreversible damage. The paucibacillary nature of the disease poses a major hurdle in early diagnosis. The management of genital TB is therefore aimed at recognizing the anatomical, physiological, and immunological aspects of the disease in its early or latent stage in order to restore fertility. Medical management with standard anti-TB drugs may not be enough to restore normalcy and may have to be supplemented by surgical correction or assisted reproduction in order to treat the infertility.